Biochem. J. (2001)355, 105–111 (Printed in Great Britain) 105
Differential regulation of expression of genes encoding uncoupling proteins
2 and 3 in brown adipose tissue during lactation in mice
Neus PEDRAZA*, Gemma SOLANES*, Roser IGLESIAS*, Manuel VA!ZQUEZ†, Marta GIRALT* and Francesc VILLARROYA*1 *Department of Biochemistry and Molecular Biology, University of Barcelona, Avda Diagonal 645, 08028-Barcelona, Spain, and†Department of Pharmacology and Therapeutic Chemistry, University of Barcelona, Avda Joan XXIII s/n, 08028-Barcelona, Spain
Thermogenic activity in brown adipose tissue (BAT) decreases during lactation ; the down-regulation of the gene encoding uncoupling protein 1 (UCP1) is involved in this process. Our studies show that UCP2 mRNA expression does not change during the breeding cycle in mice. In contrast, UCP3 mRNA is down-regulated in lactation but it recovers after weaning, in parallel with UCP1 mRNA. This leads to a decrease in the content of UCP3 in BAT mitochondria during lactation. Low-ering the energy-sparing necessities of lactating dams by decreasing litter size or feeding with a high-fat diet prevented the down-regulation of UCP1 mRNA and UCP3 mRNA. In most cases this resulted in a less marked decrease in UCP1 and UCP3 protein in BAT mitochondria owing to lactation. Fasting for 24 h caused a different response in UCP1 and UCP3 mRNA expression : it decreased UCP1 mRNA levels but had no effect on UCP3 mRNA abundance in virgin mice ; it even increased UCP3
INTRODUCTION
Brown adipose tissue (BAT) is a site of non-shivering thermo-genesis in rodents. It provides heat in response to a cold environment in small mammals as well as in the newborns of most mammalian species, including humans [1]. Rothwell and Stock [2] proposed that BAT might also be responsible for diet-induced thermogenesis in rodents. The recent development of transgenic mice with ablation of BAT confirmed this, as these mice were cold-intolerant and tended to develop obesity [3].
The thermogenic function of BAT has been attributed to the presence of the uncoupling protein 1 (UCP1), a member of the mitochondrial inner-membrane carrier family. UCP1 uncouples oxidative phosphorylation and is present only in brown adipocyte mitochondria [4]. In 1997 the genes encoding two novel un-coupling proteins, UCP2 and UCP3, were identified. UCP2 and UCP3 have a high sequence identity with UCP1 and they uncouple oxidative phosphorylation when expressed in yeast. UCP2 is almost ubiquitous, whereas UCP3 is present only in skeletal muscle and BAT [5–8]. The physiological role of UCP2 and UCP3 in energy expenditure is unclear and, for instance, UCP3 gene expression in skeletal muscle is regulated in ac-cordance with fatty acid availability rather than with thermogenic requirements [9,10]. In contrast, transgenic mice bearing a targeted disruption of the gene encoding UCP1 were, as expected, cold-intolerant ; however, they showed no signs of impairment in diet-induced thermogenesis. UCP2 mRNA expression was up-regulated, whereas UCP3 mRNA was unaltered in BAT of these
Abbreviations used : BAT, brown adipose tissue ; PPAR, peroxisome-proliferator-activated receptor ; UCP, uncoupling protein ; WY-14,643, pirixinic acid.
mRNA expression in lactating dams. These changes did not lead to modifications in UCP1 or UCP3 protein abundance. Whereas acute treatment with peroxisome-proliferator-activated receptor
(PPAR)αand PPARγagonists increased UCP1 mRNA levels
only in lactating dams, UCP3 mRNA expression was induced by both kinds of PPAR activator in lactating dams and by PPARα agonists in virgin mice. It is concluded that modifications of UCP2 mRNA levels are not part of the physiological adaptations taking place in BAT during lactation. In contrast, the down-regulation of UCP3 mRNA expression and mitochondrial UCP3 content is consistent with a role for the gene encoding UCP3 in the decrease in metabolic fuel oxidation and thermogenesis in BAT during lactation.
Key words : fibrate, mitochondria, obesity,
peroxisome-proliferator-activated receptor, troglitazone.
mice [11,12]. It was proposed that the loss of UCP1 might have been compensated for by UCP2 up-regulation [11], although further research questioned this possibility [12]. In contrast, mice with targeted disruption of the UCP3 gene did not show major metabolic abnormalities [13,14] but the overexpression of UCP3 in muscle causes decreased fat accumulation despite enhanced food intake [15]. UCP2 and UCP3 genes are subject to a complex hormonal regulation in brown adipocytes, especially by leptin, retinoids, thyroid hormones orβ
$-adrenergic agonists (re-viewed in [16]). Activators of peroxisome-proliferator-activated receptor-γ (PPARγ), such as thiazolidinediones or 15-deoxy-prostaglandin J
# induce the expression of UCP2 mRNA in
cultured brown adipocytes [17,18], whereas chronic treatments of rodents with thiazolidinediones induced the expression of both UCP2 and UCP3 mRNA in BAT [19].
106 N. Pedraza and others
thermogenic function of BAT decreases during lactation as shown by tissue hypotrophy, a decrease in mitochondrial bio-genesis and an impaired expression of the gene encoding UCP1 [26–28]. However, it is not known whether the expression of other genes encoding UCPs in BAT is altered during the breeding cycle. Here we report a study on the changes in expression of genes encoding UCP2 and UCP3 in BAT during late pregnancy, lactation and weaning. We also measured the effects on the genes encoding UCPs of direct (starvation or high-fat diet) or in-direct (changes in litter size) nutritional manipulations during the breeding cycle. Finally we examined how lactation affects the responsiveness of the genes encoding UCPs to PPAR activators.
MATERIALS AND METHODS
Adult female Swiss mice were maintained in standard conditions of light (12 h light}12 h dark cycle) and temperature (21³1°C)
and were fed with a standard diet composed of 72%
carbo-hydrate, 6%fat and 22%protein (based on percentages of gross energy) (B. K. Universal, Barcelona, Spain), unless indicated otherwise. They were mated and the day of pregnancy was determined by the presence of vaginal plugs. Litter sizes were adjusted at birth to 10 pups except when the effects of ex-ceptionally small (4 pups) or large (18 pups) litters were de-termined. Mice were studied under the following conditions : late-pregnant (day 19), lactating (days 1, 7 and 15), 24 h after abrupt weaning (pups removed after 15 days of suckling), after spontaneous weaning (on day 30 of lactation) and virgin controls. Virgin mice, pregnant dams and dams after abrupt weaning were caged in pairs and lactating dams were caged singly. The effects of fasting were determined after 24 h of suppression of food in 19-day pregnant mice, 15-day lactating dams and virgin mice. The effects of a high-fat diet during lactation were assessed by the replacement, after parturition, of regular chow with a diet composed of 36%carbohydrate, 42%
fat and 22% protein (percentages of gross energy) (Harlan
Teklad, Madison, WI, U.S.A.) and dams were studied on day 15 of lactation. Virgin mice were treated during the equivalent period (15 days) with the same high-fat diet. When indicated, mice received a single intraperitoneal injection of bezafibrate (Sigma, St Louis, MO, U.S.A.) (100µg}g body weight), pirixinic acid (WY-14,643 ; Cayman Chemicals, Ann Arbor, MI, U.S.A.) (50µg}g body weight) or troglitazone (Glaxo Wellcome, Stev-enage, Herts., U.K.) (100µg}g body weight) in 50% (v}v) DMSO in saline solution. Controls were given equivalent volumes of vehicle and mice were studied 6 h after injections. Direct observation and weighing of litters before and after the injections did not reveal major changes in lactational performance due to the treatments. Mice were killed by decapitation ; interscapular BAT was dissected out and immediately frozen in liquid nitrogen.
RNA from BAT was prepared with an affinity-column-based method (RNeasy ; Qiagen GmbH, Hilden, Germany). RNA (20µg) was denatured, subjected to electrophoresis on 0.9% formaldehyde}1.5%(w}v) agarose gels and transferred to
posi-tively charged membranes (N+; Boehringer Mannheim,
Mannheim, Germany). Ethidium bromide (0.2µg) was added to RNA samples to check for equal loading of gels and efficiency of transfer. Prehybridization and hybridization were performed at 55°C with 0.25 M Na
#HPO%(pH 7.2)}1 mM EDTA}20%(w}v) SDS}0.5%blocking reagent (Boehringer Mannheim) solution. Blots were hybridized with the cDNA species for rat UCP1, UCP2 or UCP3 as probes, as reported previously [29]. The
DNA probes were labelled with [α-$#P]dCTP by a random
quantified with Molecular Image System GS-525 (Bio-Rad, Richmond, VA, U.S.A.).
Mitochondrial preparations were obtained from interscapular BAT. Samples of mitochondrial protein were mixed with equal volumes of 2¬SDS loading buffer, incubated at 90°C for 5 min and subjected to SDS}PAGE [12% (w}v) gel]. Proteins were transferred to PVDF membranes ; immunological detection was performed with a rabbit affinity-purified UCP3 antiserum (Alpha Diagnostic, San Antonio, TX, U.S.A.) that had previously been established to detect UCP3 protein in mouse samples [33]. It was used at 4µg}ml dilution ; detection was achieved by the enhanced chemiluminescence detection system (ECL2; Amersham, Little Chalfont, Bucks., U.K.). As a positive control we used L6E9 cells transduced with an adenoviral vector driving the expression of the UCP3 cDNA (C. Garcı!a, F. Villarroya and A. M. Go! mez-Foix, unpublished work). Blots were stripped and probed with a rabbit antiserum against mouse UCP1, provided by Dr E. Rial,
and with a mouse antibody against rat cytochrome c (BD
PharMingen, Heidelberg, Germany). The sizes of the proteins detected were estimated by using protein molecular mass standards (Bio-Rad). Quantification of autoradiographs and enhanced chemiluminescence signals was performed by scanning densitometry.
Statistical analysis was performed by Student’sttest.
RESULTS
Changes in mRNA levels of UCPs in BAT from late-pregnant mice, lactating mice and dams after weaning
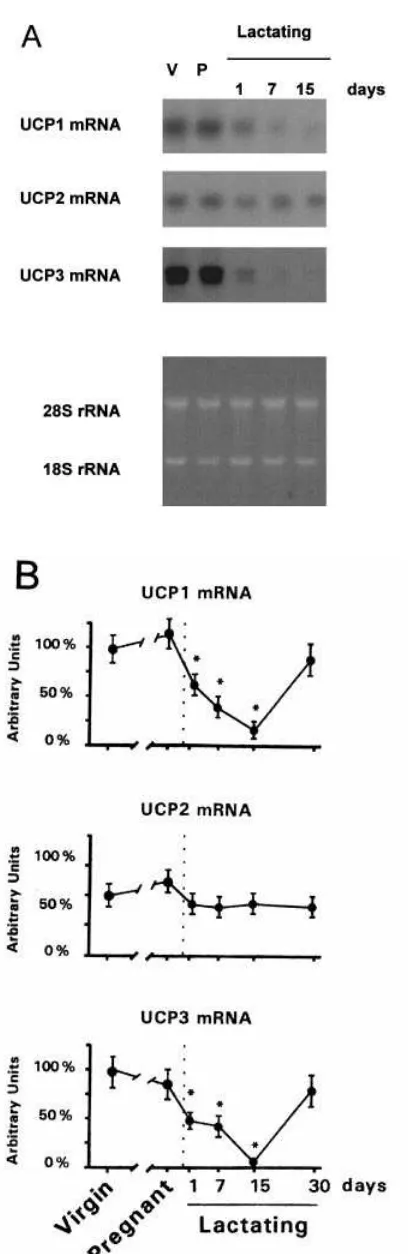
Figure 1 shows the changes in expression of the three mRNA species of UCPs in late-pregnant mice and in dams at different stages of lactation [ just after parturition (day 1), at early lactation (day 7), at mid-lactation (day 15) and after spontaneous (day 30) weaning]. UCP1 mRNA levels were not modified in late-pregnant mice ; they declined progressively during lactation and reached a minimum in mid-lactation. After spontaneous weaning, dams showed UCP1 mRNA levels similar to those in virgin controls. UCP2 mRNA levels were not significantly different from virgin controls in pregnant or lactating mice. UCP3 mRNA expression was unaltered in late-pregnant mice but declined only 1 day after parturition and was barely detectable in dams at days 7 or 15 of lactation. After spontaneous weaning, dams showed UCP3 mRNA levels similar to those in controls. The effects of abrupt weaning of 15-day lactating dams were studied and results indicated that the down-regulation of expression of UCP1 mRNA and UCP3 mRNA in BAT was completely reversed 24 h after the withdrawal of pups from lactating mothers (106³14%
for UCP1 mRNA and 118³28%for UCP3 mRNA, compared
with virgin controls ; means³S.E.M.).
Changes in the mitochondrial content of UCP1 and UCP3 in BAT from late-pregnant mice, lactating mice and dams after weaning
107
[image:3.612.74.281.45.672.2]Uncoupling-protein genes in brown adipose tissue during lactation
Figure 1 Changes in the expression of UCP1, UCP2 and UCP3 mRNAs in BAT in late-pregnant, lactating and spontaneously weaned mice
(A) Example of Northern blot analysis of RNA (20µg per lane) from interscapular BAT probed for UCP1, UCP2 and UCP3 mRNA detection (top panels) and ethidium bromide staining of the
Figure 2 Immunoblot analysis of changes in the content of UCP1 and UCP3 proteins in mitochondria from BAT in lactating and spontaneously weaned mice
(a) Western blot analysis showing UCP1 and UCP3 protein detection in BAT mitochondrial preparations from virgin controls (4µg, V), from L6E9 cells transduced with an adenoviral vector driving the expression of the UCP3 cDNA (2µg, U3) and from mice fetuses at term (35µg, F). (b) Example of immunoblot analysis of 15µg mitochondrial protein from interscapular BAT with specific antibodies against UCP1, UCP3 and cytochromec. V, virgin
controls.
agreement with the known expression of the UCP1 gene in late fetal life but the virtual absence of UCP3 mRNA from brown fat before birth [29]. These results indicate that the cross-reactivity of the anti-UCP1 and anti-UCP3 antibodies was negligible and that they could be used for specific assessment of the relative abundance of these proteins in BAT mitochondrial samples.
The content of UCP1 in BAT mitochondrial preparations was unchanged in late-pregnant mice in comparison with virgin controls (results not shown), whereas it decreased progressively from early lactation to mid-lactation (38³6%, 19³4% and 7³1%in 1-day, 7-day and 15-day lactating mice respectively, compared with virgin controls ; means³S.E.M.,P%0.05,n¯3 or 4). On day 30 of lactation, UCP1 protein levels were only partly recovered (33³6%compared with virgin controls ;P%
0.05,n¯3). Similarly, a marked decrease in UCP3 protein in mitochondria from BAT was present during lactation (19³5%, 21³4%and 18³4%in 1-day, 7-day and 15-day lactating mice respectively ;P%0.05,n¯3 or 4). Spontaneously weaned dams (day 30 of lactation) had UCP3 levels in mitochondria similar to those in virgin mice (87³14%of virgin control values). Abrupt weaning (for 24 h) of mice in mid-lactation did not significantly modify the UCP1 and UCP3 mitochondrial protein levels. Changes in the content of UCP1 and UCP3 protein were specific, because cytochrome cwas unaltered during the breeding cycle
(Figure 2).
Effects of fasting on the mRNA levels of UCPs and the mitochondrial content of UCP1 and UCP2 in BAT from late-pregnant and lactating mice
The effects of 24 h of fasting on the mRNA expression of UCPs in BAT were determined in virgin, late-pregnant or mid-lactating
*Statis-108 N. Pedraza and others
Table 1 Effects of fasting for 24 h on the abundance of UCP1, UCP2 and UCP3 mRNA species in BAT of virgin, late-pregnant and mid-lactating mice Virgin, 19-day pregnant and 15-day lactating mice were fasted for 24 h and BAT was studied. RNA (20µg) was analysed by Northern blot hybridization and results were quantified by densitometric scanning. Results are expressed as percentages of virgin control values and are means³S.E.M. for three independent experiments. *Statistically significant difference (P% 0.05) compared with the corresponding values in fed mice.
Animals Treatment
UCP1 mRNA (% of control)
UCP2 mRNA (% of control)
UCP3 mRNA (% of control) Virgin Fed 100³21 100³28 100³18
Fasted 47³8* 210³22* 91³24 Pregnant Fed 111³19 124³15 73³18 Fasted 51³16* 196³20* 117³22 Lactating Fed 7³2 78³19 6³1
Fasted 11³3 230³44* 39³8*
Table 2 Effects of litter size on the abundance of UCP1, UCP2 and UCP3 mRNA species in BAT of 15-day lactating mice
Litter sizes were adjusted at birth to 4, 10 or 18 pups and BAT from 15-day lactating dams was studied. RNA (20µg) was analysed by Northern blot hybridization and results were analysed by densitometric scanning. Results are expressed as percentages of virgin control values and are means³S.E.M. for three independent experiments. *Statistically sig-nificant difference (P%0.05) compared with virgin controls (see Table 1) ;†statistically significant difference (P%0.05) compared with dams nursing normal-sized litters (10 pups).
Litter size (number of pups)
UCP1 mRNA (% of control)
UCP2 mRNA (% of control)
UCP3 mRNA (% of control)
4 76³12† 82³22 59³7*†
10 7³1* 76³1 19³4*
18 11³4* 41³5*† 26³8*
mice (Table 1). Expression of UCP1 mRNA decreased in fasted virgin mice and the same response was found in late-pregnant mice. However, in 15-day lactating mice, which showed very low levels of UCP1 mRNA, fasting did not cause a further decrease. Fasting up-regulated UCP2 mRNA expression in BAT by approx. 2–3 fold in virgin, pregnant and lactating mice. UCP3 mRNA expression was unaltered by fasting in BAT from virgin or pregnant mice. However, it was significantly up-regulated when lactating mice were fasted, although not to the levels found in virgin controls.
No significant changes were observed in the content of UCP1 and UCP3 in BAT mitochondria owing to fasting of virgin, late-pregnant or mid-lactating mice for 24 h (results not shown).
Effects of litter size on the mRNA levels of UCPs and the content of UCP1 and UCP3 proteins in BAT from lactating mice
Dams nursing litters of different sizes showed different levels of UCP1 mRNA expression in BAT. Down-regulation of UCP1 mRNA levels owing to lactation was prevented in mice nursing only four pups (Table 2). Dams nursing 18 pups showed the same UCP1 mRNA levels as those nursing ten pups. UCP2 mRNA was altered only in dams nursing large litters ; UCP2 mRNA ex-pression in dams nursing 18 pups was approximately half of that in virgin controls. UCP3 mRNA expression in dams behaved similarly to that of UCP1 mRNA : the decrease due to lactation was partly prevented when litters were small and equally low when dams nursed normal-sized or large litters. When the content
Table 3 Effects of a high-fat diet on the abundances of UCP1, UCP2 and UCP3 mRNA species in BAT of virgin and lactating mice
Lactating mice were fed after parturition with a high-fat or a high-carbohydrate diet and studied on day 15 of lactation. Virgin mice were fed for an equivalent period (15 days) with the two different diets. RNA (20µg) was analysed by Northern blot hybridization and results were quantified by densitometric scanning. Results are expressed as percentages of virgin control values and are means³S.E.M. for four independent experiments. *Statistically significant difference (P%0.05) between groups treated with the two different diets.
Animals Diet
UCP1 mRNA (% of control)
UCP2 mRNA (% of control)
UCP3 mRNA (% of control) Virgin High-carbohydrate 100³24 100³16 100³21
High-fat 85³19 117³11 166³33 Lactating High-carbohydrate 11³3 109³21 13³2
High-fat 89³11* 155³31 45³14*
these experimental situations, the only significant change observed was that dams nursing small litters showed a less marked decrease (54³15% compared with virgins ; P%0.05,
n¯3) than dams nursing normal-sized litters. For UCP3 protein, no significant changes due to litter size were observed.
Effects of a high-fat diet on the mRNA levels of UCPs and the content of UCP1 and UCP3 proteins in BAT from virgin or lactating mice
Mice were treated with a high-fat diet from delivery to day 15 of lactation and the effects on the mRNA expression of UCPs were determined. A parallel study in virgin mice fed with the high-fat diet for the same duration (15 days) was undertaken. This diet resulted in an average increase in energy intake of 22.1% in
virgin mice and 22.5% in lactating dams. UCP1 mRNA was
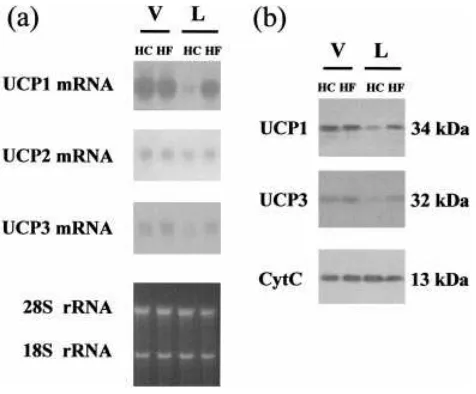
unaffected by the high-fat diet in virgin mice but was up-regulated in lactating mothers (Table 3). The down-regulation of UCP1 mRNA due to lactation was prevented almost completely by the high-fat diet. UCP2 mRNA was not affected sig-nificantly by the diet. UCP3 mRNA was not affected sigsig-nificantly by the diet in virgin mice, whereas the high-fat diet caused an increase in UCP3 mRNA expression in lactating mice compared with dams fed with the control diet. Figure 3(a) shows a represen-tative Northern blot analysis of these changes.
Neither the UCP1 nor UCP3 content in BAT mitochondria was significantly modified by the high-fat diet in virgin mice (Figure 3b). However, the relative abundances of both proteins were increased in lactating mice fed with the high-fat diet in comparison with dams fed with the high-carbohydrate diet (4.2³0.7-fold and 4.9³0.7-fold increases for UCP1 and UCP3 proteins respectively ;P%0.05,n¯3).
Effects of bezafibrate, WY-14643 or troglitazone on the mRNA levels of UCPs in BAT from virgin or lactating mice
109
Uncoupling-protein genes in brown adipose tissue during lactation
Figure 3 Representative blots of levels of UCP mRNA species and the content of UCP1 and UCP3 protein in BAT from virgin and lactating mice fed with a high-fat diet
(a) Example of Northern blot analysis of RNA (20µg per lane) from interscapular BAT probed for UCP1, UCP2 and UCP3 mRNA detection (top panels) and ethidium bromide staining of the gel showing equal loading of RNA samples (bottom panel). (b) Example of immunoblot analysis of 15µg of mitochondrial protein from interscapular BAT with specific antibodies against UCP1, UCP3 and cytochromec. V, virgin controls ; L, 15-day lactating dams ; HC, mice fed with the
[image:5.612.57.295.55.252.2]high-carbohydrate diet ; HF, mice fed with the high-fat diet.
Table 4 Effects of bezafibrate, WY-14,643 and troglitazone on the abundances of UCP1, UCP2 and UCP3 mRNA species in BAT of virgin and lactating mice
Virgin or 15-day lactating mice were injected intraperitoneally with bezafibrate (100µg/g body weight), WY-14,643 (50µg/g body weight), troglitazone (100µg/g body weight) or vehicle solution ; BAT was studied. RNA (20µg) was analysed by Northern blot hybridization and results were analysed by densitometric scanning. Results are expressed as percentages of vehicle-treated virgin mice and are means³S.E.M. for three independent experiments. *Statistically significant difference (P%0.05) between treated groups and vehicle controls in virgin or lactating mice.
Animals Treatment
UCP1 mRNA (% of control)
UCP2 mRNA (% of control)
UCP3 mRNA (% of control) Virgin Vehicle 100³11 100³18 100³14
Bezafibrate 85³18 118³21 177³20* WY-14,643 120³13 106³17 210³31* Troglitazone 91³11 85³18 117³22 Lactating Vehicle 16³5 81³18 33³6
Bezafibrate 89³11* 179³22* 95³24* WY-14,643 78³15* 166³18* 310³29* Troglitazone 55³6* 201³17* 71³22*
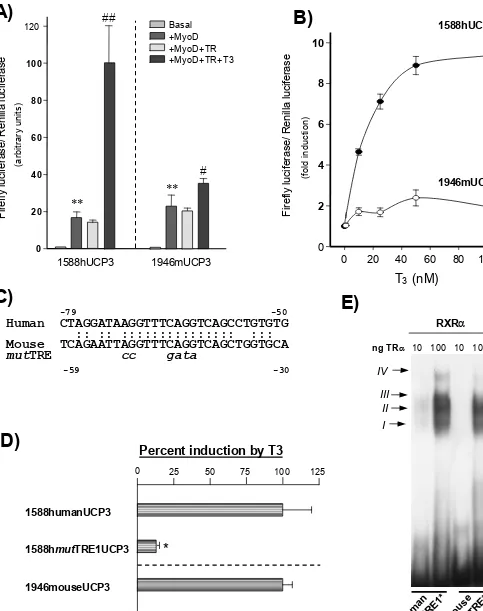
induced 10-fold by WY-14,643 and only 2–3-fold by bezafibrate or troglitazone.
DISCUSSION
We report that UCP1 mRNA is down-regulated during lactation but recovers after spontaneous or sudden weaning. This resulted in a decrease in UCP1 protein in BAT mitochondria from lactating mice and some recovery after spontaneous weaning but
findings are similar to those described previously for rats or mice, except that late-pregnant rats already show a decrease in UCP1 mRNA levels [27,34]. The decreased expression of the gene encoding UCP1 has been proposed to be involved in the decrease of BAT thermogenesis during lactation. Our results show that, whereas UCP1 and UCP3 mRNA species were down-regulated during lactation, UCP2 was not. Thus either there is a post-transcriptional regulation of gene expression or UCP2 is not involved in the regulatory metabolic modifications taking place in BAT during the breeding cycle. To answer these points, further research on the content of the UCP2 protein in mito-chondria will require the development of highly specific anti-bodies that are not yet available. In contrast, the potentially ‘ compensatory ’ up-regulation of UCP2 mRNA in mice when UCP1 mRNA expression is suppressed by targeted disruption of the gene encoding UCP1 [11] does not occur in association with the physiological suppression of UCP1 mRNA expression owing to lactation. In skeletal muscle of lactating mice, UCP3 mRNA is down-regulated [30], whereas UCP2 mRNA expres-sion is unaltered (N. Pedraza and F. Villarroya, unpublished work). However, UCP2 mRNA is up-regulated in the uterus of pregnant rats [35]. These findings indicate a tissue-specific regulation of the gene encoding UCP2 during the breeding cycle. The down-regulation of UCP3 mRNA and UCP3 protein levels during lactation and their recovery after weaning, which is similar to those for UCP1, might have various interpretations. It might be involved in the decrease in mitochondrial thermogenesis in lactation. Similarly, if UCP3 is specifically related to the fatty acid oxidation rates in mitochondria, a decrease in UCP3 content would be consistent with the low oxidation rate of fatty acids expected in BAT during lactation, when the oxidation of metabolic fuels for thermogenesis is impaired. The same reason-ing can be applied to the experiments with litters of different sizes : when litters are small, both thermogenesis and lipid oxidation in the tissue are less depressed [36] and UCP1 mRNA and UCP3 mRNA are less down-regulated, although the impact of this modification on the levels of UCP proteins is observed only for UCP1. An identical conclusion can be reached from experiments in which the energy stress due to lactation was partly prevented by feeding dams with a high-fat diet : again, the down-regulation of UCP1 and UCP3 mRNA species and mitochondrial proteins was prevented, in whole or in part. Thus a parallel was observed for the regulation of expression of the genes encoding UCP1 and UCP3 in different periods of the breeding cycle and in response to nutritional manipulations, with the sole exception of the response to fasting.
mito-110 N. Pedraza and others
mRNA levels need a longer duration for translation before they reach detectable protein levels. This has already been observed for changes in UCP3 gene expression in skeletal muscle [30].
Decreased sympathetic activity has been considered to be a major regulatory event leading to the down-regulation of the gene encoding UCP1 during lactation [38,39]. However, because sympathetic activity determines lipolysis rates and lipoprotein lipase activity [40,41] in BAT, it is difficult to assess to what extent the adrenergic regulation of gene expression in BAT might be due to a direct cAMP-dependent influence on gene promoters or to the action of lipid-derived molecules formed owing to lipid catabolism, via PPAR-responsive gene elements. We addressed the issue of whether the UCP genes were differently responsive to
PPAR agonists in io and used acute injections of PPAR
agonists with different specificities for PPAR subtypes. We observed previously that UCP1 mRNA expression is sensitive to PPARαor PPARγagonistsinioin lactating dams [42]. This is
consistent with the presence of a PPARγand PPARαresponse element in the UCP1 gene enhancer [42] and the highest sensitivity of lactating dams might be due to depressed basal levels of this signalling pathway in a physiological situation of depressed lipid catabolism and to the generation of endogenous PPAR-dependent signals. A similar observation was made for UCP2 mRNA regulation, which responds to PPARγagonists in brown adipocytes in culture [17,18]. In the present study we observed
that UCP3 mRNA expression was also responsive to PPARα
and PPARγagonists but it showed a much higher sensitivity to PPARαactivators than the genes encoding UCP1 and UCP2 :
PPARα activators induced UCP3 mRNA expression even in
virgin mice and they caused a maximal activation (10-fold induction) in lactating dams. The activation of expression of the
gene encoding UCP3 by PPARαagonists has been reported in
muscle in newborns [43] and PPARα-responsive elements are present in the human UCP3 gene promoter (G. Solanes, N. Pedraza and F. Villarroya, unpublished work). Target genes for
PPARα usually encode components of the lipid oxidation
machinery [44] ; the preferential activation of the gene encoding
UCP3 by PPARαactivators supports the involvement of UCP3
in these metabolic pathways. Thus UCP3 mRNA down-regulation during lactation might be elicited by the decreased fatty acid catabolism in BAT during this period and might be part of the metabolic adaptations associated with the decrease in fuel oxidation when energy sparing is needed.
Recently, transgenic mice with targeted disruption of the UCP3 gene have been generated, and no major disturbances have been detected in thermogenesis and fuel energy metabolism [13,14] despite some impairment in the shift from fatty acid storage to oxidation induced in muscle in response to fasting [45]. A protective role for UCP3 in relation to the mitochondrial production of reactive oxygen species when fatty acids are oxidized has been described on the basis of the phenotype of these mice [13]. Moreover, transgenic mice overexpressing UCP3 in muscle have a decreased body weight despite hyperphagia [15]. Further research will be needed to establish the physiological meaning of the adaptive impairment in expression of the gene encoding UCP3 during lactation in the light of these new insights into the role of UCP3 in mitochondrial function.
We thank Dr D. Ricquier (CEREMOD, CNRS, Meudon, France) and Dr B. Lowell (Beth Israel Hospital, Boston, MA, U.S.A.) for the UCP1 and UCP3 cDNA probes respectively, Dr E. Rial (Centro Investigaciones Biolo!gicas. Consejo Superio de Investigaciones Cientı!ficas, Madrid, Spain) for the UCP1 antibody, and the staff of the Animal Facility, Faculty of Biology, University of Barcelona, for technical support. This work was partly supported by grant PM98.0188 from Ministerio de Educacio!n
REFERENCES
1 Nicholls, D., Cunningham, S. A. and Rial, E. (1986) The bioenergetic mechanisms of brown adipose tissue. In Brown Adipose Tissue (Trayhurn, P. and Nicholls, D., eds.), pp. 86–104, Edward Arnold, London
2 Rothwell, N. J. and Stock, M. J. (1979) A role for brown adipose tissue in diet-induced thermogenesis. Nature (London)281, 31–35
3 Lowell, B. B., Susulic, V., Hamann, A., Lawitts, J. A., Himms-Hagen, J., Boyer, B. B., Kozak, L. P. and Flier, J. S. (1993) Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature (London)366, 740–742 4 Ricquier, D., Casteilla, L. and Bouillaud, F. (1991) Molecular studies of the
uncoupling protein. FASEB J.5, 2237–2242
5 Fleury, C., Neverova, M., Collins, S., Raimbault, S., Champigny, O., Levi-Meyrueis, C., Bouillaud, F., Seldin, M. F., Surwit, R. S., Ricquier, D. and Warden, C. H. (1997) Uncoupling protein-2 : a novel gene linked to obesity and hyperinsulinemia. Nat. Genet.15, 269–272
6 Gimeno, R. E., Dembski, M., Weng, X., Shyjan, A. W., Gimeno, C. J., Iris, F., Ellis, S. J., Deng, N., Woolf, E. A. and Tartaglia, L. A. (1997) Cloning and characterization of an uncoupling protein homolog : a potential molecular mediator of human thermogenesis. Diabetes46, 900–906
7 Boss, O., Samec, S., Paolini-Giacobino, A., Rossier, C., Dulloo, A., Seydoux, J., Muzzin, P. and Giacobino, J. P. (1997) Uncoupling protein-3 : a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett.408, 39–42 8 Vidal-Puig, A., Solanes, G., Grujic, D., Flier, J. S. and Lowell, B. B. (1997) UCP3 : an
uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem. Biophys. Res. Commun.235, 79–82 9 Boss, O., Bobbioni-Harch, A., Assimacopoulos-Jeannet, F., Muzzin, P., Munger, R.,
Giacobino, J. P. and Golay, A. (1998) Uncoupling protein-3 expression in skeletal muscle and free fatty acids in obesity. Lancet351, 1933
10 Brun, S., Carmona, M. C., Mampel, T., Vin4as, O., Giralt, M., Iglesias, R. and Villarroya, F. (1999) Uncoupling protein-3 gene expression in skeletal muscle during development is regulated by nutritional factors that alter circulating non-esterified fatty acids. FEBS Lett.453, 205–209
11 Enerback, S., Jacobsson, A., Simpson, E. M., Guerra, C., Yamashita, H., Harper, M. E. and Kozak, L. P. (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature (London)387, 90–94
12 Matthias, A., Jacobsson, A., Cannon, B. and Nedergaard, J. (1999) The bioenergetics of brown fat mitochondria from UCP1-ablated mice. Ucp1 is not involved in fatty acid-induced de-energization (‘ uncoupling ’). J. Biol. Chem.274, 28150–28160 13 Vidal-Puig, A. J., Grujic, D., Zhang, C. Y., Hagen, T., Boss, O., Ido, Y., Szczepanik, A.,
Wade, J., Mootha, V., Cortright, R. et al. (2000) Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem.275, 16258–16266
14 Gong, D. W., Monemdjou, S., Gavrilova, O., Leon, L. R., Marcus-Samuels, B., Chou, C. J., Everett, C., Kozak, L. P., Li, C., Deng, C. et al. (2000) Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J. Biol. Chem.275, 16251–16257
15 Clapham, J. C., Arch, J. R., Chapman, H., Haynes, A., Lister, C., Moore, G. B., Piercy, V., Carter, S. A., Lehner, I., Smith, S. A. et al. (2000) Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature (London) 406, 415–418
16 Ricquier, D. and Bouillaud, F. (2000) The uncoupling protein homologues : UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem. J.345, 161–179
17 Carmona, M. C., Valmaseda, A., Iglesias, R., Mampel, T., Vin4as, O., Giralt, M. and Villarroya, F. (1998) 9-cisretinoic acid induces the expression of the uncoupling
protein-2 gene in brown adipocytes. FEBS Lett.441, 447–450
18 Camirand, A., Marie, V., Rabelo, R. and Silva, J. E. (1998) Thiazolidinediones stimulate uncoupling protein-2 expression in cell lines representing white and brown adipose tissues and skeletal muscle. Endocrinology139, 428–431
19 Kelly, L. J., Vicario, P. P., Thompson, G. M., Candelore, M. R., Doebber, T. W., Ventre, J., Wu, M. S., Meurer, R., Forrest, M. J., Conner, M. W. et al. (1998) Peroxisome proliferator-activated receptorsγandαmediatein vivoregulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology139, 4920–4927
20 Trayhurn, P. (1983) Decreased capacity for non-shivering thermogenesis during lactation in mice. Pflu$gers Arch.398, 264–265
21 Frigerio, C., Schutz, Y., Witeshead, R. and Jecquier, E. (1992) Postprandial thermogenesis in lactating and non-lactating women from The Gambia. Eur. J. Clin. Nutr.46, 7–13
22 Trayhurn, P. (1989) Thermogenesis and the energetics of pregnancy and lactation. Can. J. Physiol. Pharmacol.67, 370–375
23 Ravussin, E. and Swinburn, B. A. (1992) Pathophysiology of obesity. Lancet340, 404–408
111
Uncoupling-protein genes in brown adipose tissue during lactation
25 Butte, N. (2000) Dieting and exercise in overweight, lactating women. New Engl. J. Med.342, 502–503
26 Trayhurn, P., Douglas, J. B. and McGuckin, M. M. (1982) Brown adipose tissue thermogenesis is ‘ suppressed ’ during lactation in mice. Nature (London)298, 59–69 27 Martin, I., Giralt, M., Vin4as, O., Iglesias, R., Mampel, T. and Villarroya, F. (1989)
Adaptative decrease in expression of the mRNA for uncoupling protein and subunit II of cytochromecoxidase in rat brown adipose tissue during pregnancy and lactation.
Biochem. J.263, 965–968
28 Martin, I., Giralt, M., Vin4as, O., Iglesias, R., Mampel, T. and Villarroya, F. (1995) Co-ordinate decrease in the expression of the mitochondrial genome and nuclear genes for mitochondrial proteins in the lactation-induced mitochondrial hypotrophy of rat brown fat. Biochem. J.308, 749–752
29 Carmona, M. C., Valmaseda, A., Brun, S., Vin4as, O., Mampel, T., Iglesias, R., Giralt, M. and Villarroya, F. (1998) Differential regulation of uncoupling protein-2 and uncoupling protein-3 gene expression in brown adipose tissue during development and cold exposure. Biochem. Biophys. Res. Commun.243, 224–228
30 Pedraza, N., Solanes, G., Carmona, M. C., Iglesias, R., Vin4as, O., Mampel, T., Vazquez, M., Giralt, M. and Villarroya, F. (2000) Impaired expression of the uncoupling protein-3 gene in skeletal muscle during lactation : fibrates and troglitazone reverse lactation-induced downregulation of the uncoupling protein-3 gene. Diabetes49, 1224–1230
31 Isseman, I., Prince, R. A., Tugwood, J. D. and Green, S. (1993) The peroxisome proliferator-activated receptor : retinoid X receptor heterodimer is activated by fatty acids and fibrate hypolipidaemic drugs. J. Mol. Endocrinol.11, 37–47
32 Yu, K., Bayona, W., Kallen, C. B., Harding, H. P., Ravera, C. P., McMahon, G., Brown, M. and Lazar, M. A. (1995) Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem.270, 23975–23983
33 Spiegelman, B. M. (1998) PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes47, 507–514
34 Trayhurn, P. and Jennings, G. (1987) Functional atrophy of brown adipose tissue during lactation in mice. Effects of lactation and weaning on mitochondrial GDP binding and uncoupling protein. Biochem. J.248, 273–276
Received 31 May 2000/4 January 2001 ; accepted 19 January 2001
35 Masaki, T., Yoshimatsu, H., Chiba, S., Kurokawa, M. and Sakata, T. (1999) Up-regulation of uterine UCP2 and UCP3 in pregnant rats. Biochim. Biophys. Acta1440, 81–88
36 Isler, D., Trayhurn, P. and Lunn, P. G. (1994) Brown adipose tissue metabolism in lactating rats : the effect of litter size. Ann. Nutr. Metab.28, 101–109
37 Champigny, O. and Ricquier, D. (1990) Effects of fasting and refeeding on the level of uncoupling protein mRNA in rat brown adipose tissue : evidence for diet-induced and cold-induced responses. J. Nutr.120, 1730–1736
38 Trayhurn, P. and Wusteman, M. C. (1987) Sympathetic activity in brown adipose tissue in lactating mice. Am. J. Physiol.253, E515–E520
39 Villarroya, F., Felipe, A. and Mampel, T. (1987) Reduced noradrenaline turnover in brown adipose tissue of lactating rats. Comp. Biochem. Physiol.86A, 481–483 40 Bukowiecki, L. J. (1986) Lipid metabolism in brown adipose tissue. In Brown Adipose
Tissue (Trayhurn, P. and Nicholls, D., eds.), pp. 105–121, Edward Arnold, London 41 Carneheim, C., Nedergaard, J. and Cannon, B. (1984) Beta-adrenergic stimulation of
lipoprotein lipase in rat brown adipose tissue during acclimation to cold. Am. J. Physiol.246, E327–E333
42 Barbera!, M. J., Schlu$ter, A., Pedraza, N., Iglesias, R., Villarroya, F. and Giralt, M. (2000) Peroxisome proliferator-activated receptorαactivates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J. Biol. Chem.276, 1486–1493 43 Brun, S., Carmona, M. C., Mampel, T., Vin4as, O., Giralt, M., Iglesias, R. and
Villarroya, F. (1999) Activators of peroxisome proliferator-activated receptor-αinduce the expression of the uncoupling protein-3 gene in skeletal muscle : a potential mechanism for the lipid intake-dependent activation of uncoupling protein-3 gene expression at birth. Diabetes48, 1217–1222
44 Schoonjans, K., Staels, B. and Auwerx, J. (1996) Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res.37, 907–925
Impaired Expression of the Uncoupling Protein-3
Gene in Skeletal Muscle During Lactation
Fibrates and Troglitazone Reverse Lactation-Induced
Downregulation of the Uncoupling Protein-3 Gene
Neus Pedraza, Gemma Solanes, M. Carmen Carmona, Roser Iglesias, Octavi Viñas, Teresa Mampel, Manuel Vazquez, Marta Giralt, and Francesc VillarroyaThe expression of uncoupling protein ( UCP) -3 mRNA in skeletal muscle is dramatically reduced during lacta-tion in mice. The reduclacta-tion in UCP-3 mRNA levels low-ers the amount of the UCP-3 protein in skeletal muscle mitochondria during lactation. Spontaneous or abrupt weaning reverses the downregulation of the UCP-3 mRNA but not the reduction in UCP-3 protein levels. In lactating and virgin mice, however, fasting increases UCP-3 mRNA levels. Changes in UCP-3 mRNA occur in parallel with modifications in the levels of free fatty acids, which are reduced in lactation and are upregulated due to weaning or fasting. Modifications in the energy nutritional stress of lactating dams achieved by manip-ulating litter sizes do not influence UCP-3 mRNA levels in skeletal muscle. Conversely, when mice are fed a high-fat diet after parturition, the downregulation of UCP-3 mRNA and UCP-3 protein levels due to lactation is partially reversed, as is the reduction in serum free fatty acid levels. Treatment of lactating mice with a sin-gle injection of bezafibrate, an activator of the peroxi-some proliferator-activated receptor ( PPAR) , raises UCP-3 mRNA in skeletal muscle to levels similar to those in virgin mice. 4-chloro-6-[( 2,3-xylidine) -pirim-idinylthio] acetic acid ( WY-14,643) , a specific ligand of the PPAR-␣subtype, causes the most dramatic increase
in UCP-3 mRNA, whereas troglitazone, a specific acti-vator of PPAR-␥, also significantly increases UCP-3
mRNA abundance in skeletal muscle of lactating mice. However, in virgin mice, bezafibrate and WY-14,643 do not significantly affect UCP-3 mRNA expression, whereas troglitazone is at least as effective as it is in lac-tating dams. It is proposed that the UCP-3 gene is reg-ulated in skeletal muscle during lactation in response to changes in circulating free fatty acids by mechanisms involving activation of PPARs. The impaired expression of the UCP-3 gene is consistent with the involvement of
UCP-3 gene regulation in the reduction of the use of fatty acids as fuel by the skeletal muscle and in impaired adaptative thermogenesis, both of which are major metabolic adaptations that occur during lacta-tion. Diabetes49:1224–1230, 2000
U
nco upling pro tein (UCP)-2 and UCP-3 are 2 recently cloned genes that have a high sequence homology with the brown adipose tissue UCP-1. They uncouple oxidative phosphorylation when transfected into yeast, and, by analogy with UCP-1, they are considered to be potentially involved in regulatory thermo-genesis (1–4). The UCP-2 and UCP-3 genes are located adja-cent in a region of human chromosome 11, which coincides with quantitative trait loci for obesity and type 2 diabetes (5). The UCP-3 gene has attracted attention due to its preferential expression in thermogenic tissues, brown fat, and skeletal muscle in rodents and skeletal muscle in humans. However, recent studies indicate that the physiological role of UCP-3 as a mediator of thermogenesis in muscle is quite complex. For example, UCP-3 mRNA abundance is unaltered in situations o f enhanced regulato ry thermo genesis in muscle, such as long-term cold exposure (6), whereas UCP-3 mRNA is upreg-ulated in situations of depressed muscle thermogenesis, such as starvation (7,8). In fact, most of the physiological or patho-lo gical situatio ns repo rted to date in which UCP-3 gene expression in skeletal muscle is altered (i.e., during instances of high-fat diets, postnatal development, or streptozotozin-induced diabetes) are associated with parallel changes in cir-culating free fatty acids (9–11). Fatty acids themselves have been reported to upregulate UCP-3 gene expression in mus-cle (7,12), most likely by activating peroxisome proliferator-activated receptor (PPAR)-␣(12), and it has been proposed that UCP-3 could be specifically involved in the regulation of the use o f lipids as fuel substrates in skeletal muscle. Decreased UCP-3 mRNA expression has been described in the muscle of type 2 diabetic patients (13), a situation associated with decreased rates of lipid oxidation by skeletal muscle (14), although other studies did not confirm this finding (15). Mo reo ver, several studies in o bese and type 2 diabetic humans show an association between polymorphisms in theFro m the Departments o f Bio c hemistry and Mo lec ular Bio lo gy ( N.P., G.S., M.C.C., R.I., O.V., T.M., M.G., F.V.) and Pharmac o lo gy and Therapeutic Chemistry ( M.V.) , University o f Barc elo na, Barc elo na, Spain.
Address c o rrespo ndenc e and reprint requests to Franc esc Villarro ya, PhD, Department o f Bio c hemistry and Mo lec ular Bio lo gy, University o f Barc elo na, Avda Diago nal 645, 08028 Barc elo na, Spain. E-mail: go mbau@ po rtho s.bio .ub.es.
Rec eived fo r public atio n 8 No vember 1999 and ac c epted in revised fo rm 13 Marc h 2000.
N. PEDRAZA AND ASSOCIATES
The breeding cycle is a physiological situation associated with dramatic metabolic adaptations and changes in energy balance. A major increase in energy requirements occurs dur-ing lactation due to the needs of milk production. Well-nour-ished women meet most of the additional energy costs of lac-tation by increasing food intake, and there is little evidence of energy-sparing adaptations involving basal metabolic rate or adaptative thermogenesis (18). However, a reduction in diet-induced thermogenesis has been demonstrated in lactating women under nutritionally unfavorable conditions (19), and it has been interpreted as a way to save energy in periods when food restriction overlaps with a high-energy output because of lactation. The molecular mechanisms for this adaptation are no t kno wn, but skeletal muscle has been repo rted to be involved (20). In rodents, an overall increase in the efficiency of energy utilization and a reduction in adaptative thermoge-nesis develop in lactation, even in well-nourished animals (21). Functional atrophy of brown fat, a major thermogenic tis-sue in rodents, and a reduced expression of the UCP-1 gene take place during lactation (22,23), and these have been pro-posed to contribute to energy sparing during this period. Lac-tation is a unique physiological situation in which an adapta-tive decrease in energy expenditure appears in association with hyperphagia. This pheno meno n also o ccurs in many cases of obesity, and lactation constitutes an excellent model to assess the molecular mechanisms that determine adapta-tive changes in energy expenditure and inter-organ metabolic partitioning. During lactation, metabolic adaptations develop to promote the utilization of metabolic fuels by the mammary gland. In lactating mothers, glucose and fatty acids are chan-neled to the mammary gland fo r the synthesis o f milk, whereas other tissues, including skeletal muscle, reduce their use of metabolic fuels for oxidation (24).
Here, we report that the expression of the UCP-3 gene is suppressed in the skeletal muscle o f lactating mice, even though UCP-3 mRNA expression remains highly sensitive to induction by physiological (fasting, weaning) or pharmaco-logical (PPAR activators) stimuli.
RESEARCH DESIGN AND METHODS
Materials.Bezafibrate was ac quired fro m Sigma (St. Lo uis, MO) and 4c hlo ro -6-[( 2,3-xylidine) -pirimidinylthio ]ac etic ac id ( WY-14,643) was ac quired fro m Cayman Chemic als (Ann Arbo r, MI). Tro glitazo ne was pro vided by Glaxo Well-c o me (Greenfo rd, U.K.).
Animals.Adult female Swiss mic e were used. They were maintained under standard c o nditio ns o f illuminatio n ( 12-h light/dark c yc le) and temperature (21 ± 1°C) and were fed a standard diet co mpo sed o f 72% (in gro ss energy) car-bo hydrate, 6% fat, and 22% pro tein ( B.K. Universal, Barc elo na, Spain) , unless o therwise indic ated. Female mic e were mated with adult males, and the day o f pregnanc y was determined by the presenc e o f spermato zo a in vaginal smears. When lac tating mic e were studied, litter sizes were adjusted at birth to 10 pups, exc ept when the effec ts o f exc eptio nally small ( 4 pups) o r large ( 18 pups) litters were determined. Pregnant ( day 19) , lac tating ( days 1, 7, 15, and 30) , abruptly weaned dams ( 24 h after remo val o f 15-day lac tating pups) , and virgin c o ntro l mic e were studied in basal c o nditio ns. The effec ts o f fast-ing were determined after 24-h suppressio n o f fo o d to 15-day lac tatfast-ing dams and virgin c o ntro ls. The effec ts o f a high-fat diet during lac tatio n were assessed by replac ing the standard diet by a diet c o mpo sed o f 36% ( in gro ss energy) c arbo hydrate, 42% fat, and 22% pro tein ( Harlan Teklad, Madiso n, WI) after parturition, and dams were studied on day 15 of lactation. When indicated, mic e were treated with a single intraperito neal injec tio n o f bezafibrate ( 100 µg/g bo dy wt), WY-14,643 (50 µg/g bo dy wt), o r tro glitazo ne (100 µg/g bo dy wt) in 50% dimethyl sulfo xide/saline. Co ntro ls were given equivalent vo lumes o f the vehic le, and mic e were studied 6 h after injec tio ns. Direc t o bservatio n and weighing o f litters befo re and after the injec tio ns did no t reveal majo r c hanges in lac tatio nal perfo rmanc e due to the 6-h treatments. Mic e were killed by
experiments with PPAR ac tivato rs, in whic h injec tio ns were perfo rmed at the beginning o f the light c yc le and animals were killed 6 h later. The gastro c ne-mius, extenso r digito rum lo ngus, and tibialis anterio r skeletal musc les were dissec ted and blo o d was c o llec ted. Skeletal musc le fro m mo use fetuses and spleen fro m virgin c o ntro ls were also o btained fo r c o mparative purpo ses in immuno blo t assays o f mito c ho ndrial pro teins.
RNA isolation and Northern blot hybridization.RNA fro m skeletal mus-cles was prepared using a guanidine thio cyanate metho d (25). RNA (20 µg) was denatured, electrophoresed on 1.5% formaldehyde-agarose gels, and transferred to po sitively c harged membranes (N+; Bo ehringer Mannheim, Mannheim, Ger-many) . Ethidium bro mide ( 0.2 µg) was added to RNA samples to c hec k equal lo ading o f gels and transfer effic ienc y. Prehybridizatio n and hybridizatio n were perfo rmed at 55°C using 0.25 mo l/l Na2HPO4( pH 7.2) , 1 mmo l/l EDTA, 20% SDS, and 0.5% blo c king reagent ( Bo ehringer Mannheim) so lutio n. Blo ts were hybridized using as pro bes the human c DNA fo r UCP-3 ( 4) and the mo use cDNA fo r the mito cho ndrial geno me–enco ded cyto chro me o xidase sub-unit II ( COII) ( 26) that was used as a c o ntro l. The DNA pro bes were labeled with an [␣-32
P]dCTP using a rando m o ligo nuc leo tide-primer metho d. Hybridizatio n signals were quantified using Mo lec ular Image System GS-525 ( Bio -Rad, Ric hmo nd, CA) .
Immunoblot analysis.Mito c ho ndria were iso lated fro m gastro c nemius and who le skeletal musc le fro m the legs o f fetuses and fro m adult mo use spleens (27). Samples c o ntaining 20 µg mito c ho ndrial pro tein were mixed with equal vo lumes o f 2 ⫻SDS lo ading buffer, inc ubated at 90°C fo r 5 min, and elec -tro pho resed o n SDS/12%-po lyac rylamide gels. Pro teins were transferred to po lyvinylidene difluo ride membranes, and immuno lo gic al detec tio n was per-fo rmed using a rabbit affinity-pure UCP-3 antiserum (Alpha Diagno stic , San Anto nio , TX). This antibo dy was generated against a 17–amino ac id peptide sequence located at the second and third transmembrane domain of human UCP-3, sho wing 71% ho mo lo gy with mo use UCP-3 and no signific ant ho mo lo gy with UCP-1 o r UCP-2. It was used at a 4-µg/ml dilutio n, and detec tio n was ac hieved using the enhanc ed c hemiluminesc enc e (ECL) detec tio n system (Amersham, Amersham, U.K.). As a positive control, 293 cells stably transfected with a tetra-c ytetra-c line-indutetra-c ible (Tet-On; Clo ntetetra-c h, Palo Alto , CA) tetra-c o nstrutetra-c t driving the lo ng iso fo rm o f human UCP-3 (UCP-3L) c DNA expressio n (293-U3 c ells) were used (B. Sibille, G.S., F.V., unpublished data). Blo ts were stripped thereafter and pro bed with a rabbit antiserum against bo vine heart adenine nuc leo tide translo c ase ( ANT) , a gift o f Dr. G. Brando lin ( DBMS/Bio c himie, Greno ble, Franc e), whic h, ac c o rding to previo usly established pro c edures (27), was used as a control of equal abundance of mitochondrial membrane protein in the sam-ples. The sizes o f the pro teins detec ted were estimated using pro tein mo lec u-lar-mass standards (Bio -Rad). Quantitatio n o f auto radio graphs and ECL signals was perfo rmed by sc anning densito metry.
Serum free fatty acid levels were quantified using a colorimetric acyl-CoA syn-thase and acyl-Co A o xidase-based metho d (Wako Chemicals, Neuss, Germany).
Statistic al analysis was perfo rmed with Student’sttest.
RESULTS
substi-UNCOUPLING PROTEIN-3 GENE IN LACTATION
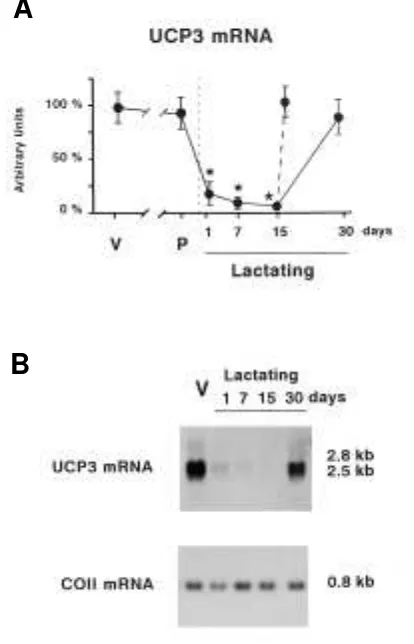
returned to control values. However, the effects of weaning on UCP-3 mRNA did not require a long duration. When 15-day lactating dams were abruptly separated from their offspring fo r 24 h, UCP-3 mRNA abundanc e was upregulated and reached levels similar to those of controls. The reduction in the expressio n o f UCP-3 mRNA during lac tatio n and the upregulation due to weaning occurred in parallel for the 2.5-and 2.8-kb mRNA species of UCP-3 (Fig. 1B). All of these changes occurred without modifications in the mRNA levels for COII, which were used as a control of overall mitochon-drial biogenesis.
UCP-3 abundance is reduced in skeletal muscle mito-chondria from lactating mice. To assess whether the reduction in UCP-3 mRNA expression results in a decrease in the relative amount of UCP-3 protein in skeletal muscle mito c ho ndria fro m lac tating mic e, immuno blo t assays o f UCP-3 were perfo rmed using a spec ific antibo dy (see
RESEARCH DESIGN AND METHODS). Analysis o f mito c ho ndrial
[image:10.612.313.547.65.394.2]detection of a single 34-kD band. 293 cells stably transfected with a tetracycline-inducible construct driving the expression of human UCP-3L. cDNA showed no signal (noninduced) or a strong (tetracycline-induced) 34-kD signal, the same size as that observed in muscle samples (Fig. 2B). No 34-kD signal was detected in mitochondria from fetal muscle or spleen in whic h UCP-2 mRNA but no t UCP-3 mRNA is expressed (6,12). This confirms the specificity of UCP-3 detection. Fig-ure 2Bshows an immunoblot analysis of UCP-3 protein lev-els in mitochondria isolated from the gastrocnemius muscle o f virgin o r lac tating mic e at different days o f lac tatio n. Homogeneity of mitochondrial preparations of muscles from virgin and lactating mice was assessed as equal signals in immuno blo t analysis o f the ANT, a mito cho ndrial pro tein
FIG. 1. Changes in UCP-3 mRNA levels in the gastrocnemius skeletal muscle of late pregnant and lactating mice. A: Representation of the abundance of UCP-3 mRNA in virgin control mice ( V) , 19-day pregnant mice ( P) , and mice at the indicated days of lactation. Discontinuous line indicates abrupt weaning of 15-day lactating dams. Points are means ± SE ( indicated by bars) of the hybridization intensity signals of 3–4 samples. The 2.8- and 2.5-kb transcripts for UCP-3 were addi-tively quantified as a single point. Data are expressed as the per-centage relative to the virgin control value. Statistical comparisons between virgin controls and experimental groups are shown by *P≤
0.05. B: Representative Northern blot analysis of 20 µg/lane of RNA
from gastrocnemius. The sizes of the UCP-3 and COII transcripts are depicted to the right.
FIG. 2. Changes in mitochondrial UCP-3 abundance in gastrocnemius skeletal muscle during lactation. A: Abundance of UCP-3 in virgin
control mice ( V) and mice at the indicated days of lactation. Bars are means ± SE of the Western blot intensity signals of 3–4 samples. Data are expressed as the percentage relative to the virgin control value. Statistical comparisons between virgin controls and experimental groups are shown by *P≤0.05. B: Representative immunoblot analy-sis of the UCP-3 content in mitochondrial preparations of gastrocne-mius from virgin mice ( V) , mice at the indicated days of lactation, and mitochondria from 18-day fetal mouse skeletal muscle ( fetus) or adult spleen. 293-U3 lanes are mitochondria preparations from 293 cells sta-bly transfected with a tetracycline-inducible human UCP-3L cDNA construct nonexposed ( –) or exposed ( +) to tetracycline. Of mito-chondrial protein, 20 µg was loaded in each lane, except for 293 cells ( 10 µg) , and was immunoblotted using a rabbit anti–UCP-3 antibody ( upper lane) and, subsequently, a rabbit anti-ANT antibody as a con-trol ( lower lane) . The sizes of the signals obtained are shown to the right of the lanes.
A
B
A
[image:10.612.53.260.67.389.2]N. PEDRAZA AND ASSOCIATES
unpublished data). A significant decrease in UCP-3 protein lev-els was achieved on days 7 and 15 of lactation, and sponta-neously weaned 30-day lactating dams already showed lower UCP-3 protein levels than those of virgin controls (Fig. 2A). 24-h weaning of 15-day lactating dams did not reverse UCP-3 protein downregulation, which remained at 44 ± 11% of vir-gin control values.
Effects of fasting on UCP-3 gene expression in skele-tal muscle of lactating mice.The effects of 24-h starvation on UCP-3 mRNA in gastrocnemius from virgin and lactating mice were determined to assess whether the impairment in UCP-3 mRNA expressio n o f lac tating mic e affec ts their response to fasting. Fasting of female virgin mice caused a significant rise in UCP-3 mRNA expression (Fig. 3), which is in agreement with previous reports (6,28). Although the basal levels of UCP-3 mRNA were very low in lactating dams, after 24 h of fasting, they rose dramatically to similar levels to those in fasted virgin mice. Thus, there was a >50-fold induc-tion of UCP-3 mRNA in gastrocnemius from fasted lactating dams compared with fed lactating controls. The same behav-ior was observed for extensor digitorum longus and tibialis anterior muscles (data not shown). COII mRNA levels were essentially unaltered due to fasting in any of the experimen-tal groups. However, the relative content of UCP protein in mitochondrial preparations of either fasted virgin or fasted lactating mice, as compared with fed animals, was unaltered: 115 ± 25% in fasted vs. fed virgins and 52 ± 12% in fasting vs. fed lactating dams.
Changes in litter size modify energy nutritional stress in lactating mice but do not affect UCP-3 mRNA expression in skeletal muscle.As an experimental approach to assess the role of the nutritional stress during lactation in the UCP-3 gene do wnregulatio n in muscle, litter sizes were manipu-lated. Litters were adjusted after birth to a low size (4 pups) or a high size (18 pups) as a way to decrease or increase, respectively, the output of milk by lactating dams. This exper-imental model is known to raise or lower, respectively, non-shivering thermogenesis and brown adipose tissue thermo-genic activity in lactating mothers (29). The impact of litter
15 of lactation, the total weight of the litters consisting of 4 pups was 45.6 ± 1.2 g, 91% of the dam’s body weight, whereas it was 108.0 ± 3.6 g in litters consisting of 18 pups, 216% of the dam’s weight. However, UCP-3 mRNA expression in skeletal muscle of dams was not significantly altered by these changes in litter size. UCP-3 mRNA levels in dams nursing small or large litters were 128 ± 23 and 106 ± 19%, respectively, vs. lac-tating controls. It is concluded that modulation of overall energy nutritional stress is not a major determinant of UCP-3 mRNA expression during lactation.
A high-fat diet during lactation partially reverses UCP-3 mRNA and UCP-3 protein downregulation in skeletal muscle.As a second approach to assess the role of nutrition on UCP-3 gene downregulation in skeletal muscle during lactation, dams were exposed after parturition to a high-fat diet and studied at mid-lac tatio n. This treatment resulted in an average increase in calo ric intake o f 22.5% with respect to lactating mice fed a regular high-carbohy-drate diet. The weight of pups from 15-day lactating mice fed a high-fat diet was higher than that of control pups (high-fat diet 8.43 ± 0.4 g, controls 6.9 ± 0.2 g, P≤0.05), thus indicat-ing the impact of this dietary treatment on the amount of energy delivered as milk by lactating dams. UCP-3 mRNA lev-els in skeletal muscle of mid-lactating dams fed the high-fat diet were significantly higher (451 ± 59%, P≤0.05) than mid-lactating mice fed a regular diet, although they did not reach the levels fo und in virgin c o ntro ls. Similar behavio r was o bserved fo r UCP-3 pro tein: the relative co ncentratio n in lactating dams fed the high-fat diet was higher (164 ± 21%, P≤
0.05) than that in lactating dams fed a regular diet.
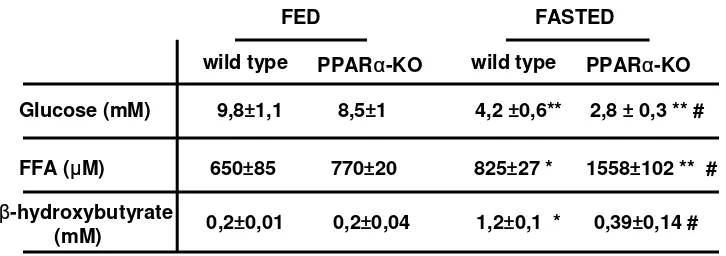
Serum nonesterified fatty acid levels in pregnant and lactating mice: the effects of weaning, fasting, high-fat diet, and changes in litter size.Table 1 shows the changes in serum free fatty acids due to the different physiological and nutritio nal situatio ns studied. Late pregnant mice did no t sho w signific ant c hanges in serum free fatty ac id levels, whereas a progressive decrease in serum free fatty acid
[image:11.612.64.295.78.223.2]con-FIG. 3. Effects of 24-h fasting of virgin and 15-day lactating mice on UCP-3 mRNA expression in gastrocnemius muscle. Bars are means ± SE of the hybridization intensity signals of at least 3 samples. Data are expressed as the percentage relative to the fed virgin value. Statisti-cal significance of comparisons between fed and fasted groups for each experimental situation are shown by *P≤0.05.
TABLE 1
Free fatty acid levels in serum o f virgin co ntro l, late pregnant, and mid-lac tating mic e under different experimental c o nditio ns
Serum free fatty
Animals ac id levels ( µmo l/l)
Virgin c o ntro l mic e
Baseline 1,278 ± 96
+ 24-h fasting 1,779 ± 113*
Late pregnant mic e
Day 19 972 ± 174
Mid-lac tating mic e
Day 1 763 ± 102*
Day 7 494 ± 79*
Day 15 411 ± 14*
+ 24-h Fasting 729 ± 110*†
+ 24-h Weaning 1,017 ± 127†
+ High-fat diet 820 ± 134*†
Lo w litter size ( 4 pups) 534 ± 133* Large litter size ( 18 pups) 525 ± 101*
Day 30 892 ± 98†
[image:11.612.327.577.95.285.2]UNCOUPLING PROTEIN-3 GENE IN LACTATION
serum free fatty acids were attained in mid-lactation; this finding is in agreement with previous reports (30). Both 24 h after abrupt weaning of mid-lactating dams or after sponta-neous weaning (day 30 of lactation), serum free fatty acid lev-els rose to levlev-els that were not significantly different from those in virgin controls. Fasting induced a rise in serum free fatty acid concentrations in virgin and mid-lactating mice. Lit-ter size had no effect on serum free fatty acids. Feeding dams a high-fat diet during lactation resulted in levels of serum free fatty acids higher than those in lactating dams fed a regular diet. In conclusion, there was a close positive association between changes in serum free fatty acid levels elicited by lac-tation and nutritional manipulations and changes in UCP-3 mRNA levels in skeletal muscle.
Differential effects of fibrates and troglitazone on UCP-3 mRNA expression in skeletal muscle of lactating mice.The present results suggest that free fatty acids may be responsible for adaptative changes in UCP-3 gene expression in skeletal muscle during lactation, and we have reported recently that fibrates, which are activato rs o f PPAR, may mediate free fatty acid effects on the UCP-3 gene (12). The effects of single injections of the hypolipidemic drug bezafi-brate, a preferential PPAR-␣ ac tivato r (31), WY-14,643, a highly specific activator of PPAR-␣(32), and troglitazone, a thiazolidinedione specific for PPAR-␥(33), were studied in mid-lactating mice (Fig. 4). Bezafibrate or WY-14,643 admin-istration did not significantly alter UCP-3 mRNA expression in skeletal musc le o f virgin mic e, whereas tro glitazo ne induced it. When injected into lactating mice, bezafibrate induced UCP-3 mRNA levels and almost reversed the down-regulation of UCP-3 gene expression in lactation. WY-14,643 caused the most dramatic increase in UCP-3 mRNA abun-dance in lactating dams, which achieved levels even higher than those in virgin controls. Troglitazone increased UCP-3 mRNA expression in skeletal muscle of lactating mice to a similar extent as that in virgin mice (3- to 4-fold induction in
injections with the PPAR activators did not significantly mod-ify serum nonesterified fatty acid levels in either virgin or lac-tating mice (data not shown).
Unaltered PPAR-␣mRNA expression in skeletal muscle
of lactating mice. Considering the different sensitivity to fibrates and particularly to PPAR-␣–specific activation in vir-gin and lactating mice, PPAR-␣gene expression in skeletal muscle of lactating mice was determined in comparison with virgin controls. Northern blot analysis showed that PPAR-␣
gene was expressed in mouse skeletal muscle as a single tran-script of 8.5 kb, as already reported (12). No difference was observed in the levels of PPAR-␣mRNA expression in gas-trocnemius skeletal muscle between virgin and 15-day lactating mice. Densitometric scanning of 4 independent Northern blot assays indicated that PPAR-␣mRNA signals in mid-lactating mice was 92 ± 28% of those found in virgin controls.
DISCUSSION
In the present study, a dramatic reduction in UCP-3 mRNA expression resulting in a decrease in the relative abundance of UCP-3 in the skeletal muscle mitochondria is described as part of the metabolic regulatory events that take place in skeletal muscle during lactation. The comparison of changes in UCP-3 mRNA and UCP-3 protein levels indicates that, as a general rule, long-term modifications in UCP-3 mRNA, such as those observed in lactation or high-fat diet, lead to signi-ficant changes in UCP-3 protein abundance in mitochondria. However, short-term modifications in UCP-3 mRNA levels, such as those elicited by 24-h fasting or 24-h weaning, had no significant effect on UCP-3 protein abundance. Indeed, pre-vio us repo rts o n the effec ts o f fasting sho wed a mo dest increase in UCP-3 protein only after 2-day starvation (28). In the absence of UCP-3 protein turnover studies, these results indicate a slow-acting translational and/or posttranslational regulation of the UCP-3 gene.
In the context of the current debate on the physiological role of UCP-3, two physiological events occur in lactation that are c o mpatible with the do wnregulatio n o f UCP-3 gene expression: a reduction in nonshivering thermogenesis (21) and a decrease in the utilization of fatty acids by muscle, which favors the use of these substrates by the mammary gland for milk production (24). The time-course of UCP-3 pro-tein downregulation throughout lactation fits well with the adaptative reduction in nonshivering thermogenesis, which develops progressively as lactation proceeds (21–23). Thus, UCP-3 protein abundance is slightly decreased in dams just after parturition, when milk production is very low, whereas it attains minimal levels in mid-lactation when milk produc-tion is maximal. However, the drop in UCP-3 mRNA after par-turition is abrupt and UCP-3 mRNA expression is extremely low in lactating mice at any moment of lactation, indicating that regulato ry events leading to a lo w expressio n o f the UCP-3 gene start lo ng befo re substantial energy-sparing mec hanisms are required. In this sense, c irc ulating fatty acids are already reduced just 1 day after parturition.
[image:12.612.34.281.58.222.2]Our results indicate that different stages of lactation or nutritio nal manipulatio ns in the breeding perio d (fasting, weaning, and high-fat diet) were associated with changes in UCP-3 mRNA expression in skeletal muscle only when the lev-els of free fatty acids were modified in the same direction and regardless o f the lipo lytic ac tivity o f adipo se tissue. Fo r
N. PEDRAZA AND ASSOCIATES
expression in skeletal muscle of lactating dams because they cause an increase in free fatty acid levels. However, lipolysis is activated during fasting, but it is reduced in weaning, when the rise in free fatty acids is caused by the sudden impairment of their use by the mammary gland (34). This supports the notion that fatty acids themselves regulate the expression of the UCP-3 gene in skeletal muscle during lactation. Other hor-mo nal o r metabo lic signals previo usly repo rted to induce the UCP-3 gene in musc le, suc h as leptin o r thyro id ho r-mones, are unlikely to play a major role in UCP-3 downreg-ulation during lactation. The levels of circulating leptin are unaltered during lactatio n (35), and, altho ugh lactatio n is associated with a mild hypothyroid state, changes in serum thyroid hormones due to fasting (36) or litter size manipula-tio n (37) do no t c o rrelate with c hanges in UCP-3 mRNA expression in muscle.
Activators of PPAR reverse the UCP-3 mRNA downregula-tion during lactadownregula-tion, and they are likely to mimic the positive action of fatty acids on the UCP-3 gene. Whereas the effects of long-term treatments with fibrates or thiazolidinediones may rely on indirect metabolic mechanisms (38,39), the effec-tiveness of PPAR activators in the very short-term exposure shown here suggests direct effects on skeletal muscle. The abil-ity of fibrates to induce the UCP-3 gene in muscle has been reported in newborn mice (12) and adult rats (39), and the highest potency of WY-14,643, a specific ligand of PPAR-␣in lactating mice, supports a major involvement of this PPAR sub-type in UCP-3 gene regulation in skeletal muscle. According to the present results, the higher sensitivity to PPAR-␣ acti-vation of the UCP-3 gene expression in skeletal muscle of lactating mice was no t attributable to changes in PPAR-␣
receptor expression. A potential explanation for these findings would be that if PPAR-␣activation mimics the effect of free fatty acids on UCP-3 mRNA in skeletal muscle (12), then this pathway of stimulation would already be quite active in virgin mice because virgin mice have higher levels of serum free fatty acid levels and UCP-3 mRNA expression. As a result, a lack of sensitivity to PPAR-␣activators is observed here. In most cases, target genes of PPAR-␣regulation code for enzymes and proteins that are part of the lipid oxidation machinery of the cell (40), and the identification of the UCP-3 gene as a target of PPAR-␣activation further supports its putative involve-ment in the regulation of fatty acid oxidation. Troglitazone, an antidiabetic thiazo lidinedio ne and a specific activato r o f PPAR-␥ (33), could also induce UCP-3 gene expression in skeletal muscle of lactating mice and normalize UCP-3 mRNA levels, but, in contrast to fibrates, it was also effective in vir-gin animals. This finding indicates a different pathway of reg-ulation of UCP-3 gene expression by PPAR-␥activation, with respect to the PPAR-␣–dependent pathway. Moreover, it sug-gests that the PPAR-␥–dependent activatio n o f the UCP-3 gene is not related to the physiological action of fatty acids pro-mo ting UCP-3 gene expressio n. Altho ugh PPAR-␥ is extremely low in skeletal muscle (12,33), chronic treatments of humans or rodents with troglitazone are known to improve insulin sensitivity and reduce triacylglyceride co ntent in skeletal muscle (41). Several recent reports claim there is a direct effect of thiazolidinediones on this tissue (42,43), which wo uld be co nsistent with the effects o n the UCP-3 gene observed in this study. However, newborn mice, which are highly sensitive to upregulation of UCP-3 gene expression in
action of thiazolidinediones (12). Further research will be necessary to establish the precise mechanisms of action of thi-azolidinediones on the UCP-3 gene in muscle and how they are modified in different physiological situations.
Insulin resistance of skeletal muscle is fundamental to the development of type 2 diabetes, and excessive exposure of muscle to free fatty acids appears to play a prominent role in the appearance of the insulin-resistant state associated with obesity (44). Understanding the regulatory mechanisms of fatty acid oxidation by the skeletal muscle cells is essential for the development of strategies to avoid an insulin-resistant state. In the present work, we report that impaired UCP-3 gene expression in skeletal muscle is a physiological event associated with the metabolic adaptations of lactation and, par-ticularly, with the reduced utilization of fatty acids by the tis-sue as an energy source. Both fibrates and troglitazone reverse the downregulation of the UCP-3 mRNA expression. Further research should be undertaken to determine the involvement of the UCP-3 gene upregulation in skeletal muscle in the hypolipidemic and antidiabetic effects of these drugs.
ACKNOWLEDGMENTS
This study was suppo rted by grants PB95.0969 and PM98.0188 from the Ministry of Education and Culture, Spain and Fundació la Marató de TV3.
Technical support by the staff of the Animal Facility and the Faculty of Biology of the University of Barcelona is acknowl-edged.
We thank Dr. B. Lowell (Beth Israel Hospital, Boston, MA), Dr. N. Glaichenhaus (University of Nice, Nice, France), and Dr. S. Green (Zeneca, Cheshire, U.K.) for the UCP-3, COII, and PPAR-␣ c DNA pro bes. We also thank Dr. G. Brando lin (DBMS/Bio chimie, CENG, Greno ble, France) fo r the anti-serum against ANT.
REFERENCES
1. Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH: Uncoupling pro-tein-2: a no vel gene linked to o besity and hyperinsulinemia. Nat Genet 15:269–272, 1997
2. Gimeno RE, Dembski M, Weng X, Shyjan AW, Gimeno CJ, Iris F, Ellis SJ, Deng N, Woolf EA, Tartaglia LA: Cloning and characterization of an uncoupling pro-tein homolog: a potential molecular mediator of human thermogenesis. Dia-betes46:900–906, 1997
3. Bo ss O, Samec S, Pao lini-Giac o bino A, Ro ssier C, Dullo o A, Seydo ux J, Muzzin P, Giacobino JP: Uncoupling protein-3: a new member of the mito-chondrial carrier family with tissue-specific expression. FEBS Lett408:39–42, 1997
4. Vidal-Puig A, Solanes G, Grujic D, Flier JS, Lowell BB: UCP-3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal mus-cle and brown adipose tissue. Biochem Biophys Res Commun235:79–82, 1997 5. Solanes G, Vidal-Puig A, Grujic D, Flier JS, Lowell BB: The human uncoupling protein-3 gene: genomic structure, chromosomal localization, and genetic basis for short and long form transcripts.J Biol Chem272:25433–25436, 1997 6. Gong DW, He Y, Karas M, Reitman M: Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, 3-adrenergic agonists, and lep-tin.J Biol Chem272:24129–24132, 1997
7. Weigle DS, Selfridge LE, Schwartz MW, Seeley RJ, Cummings DE, Havel PJ, Kuijper JL, BertrandelRio H: Elevated free fatty acids induce uncoupling pro-tein 3 expression in muscle: a potential explanation for the effects of fasting. Diabetes47:298–302, 1998
8. Millet L, Vidal H, Andreelli F, Larrouy D, Riou JP, Ricquier D, Laville M, Lan-gin D: Increased uncoupling protein-2 and -3 mRNA expression during fast-ing in obese and lean humans. J Clin Invest100:2665–2670, 1997