Progressing the analysis of Improvised Explosive Devices: Comparative study for trace detection of explosive residues in handprints by Raman spectroscopy and liquid chromatography
Texto completo
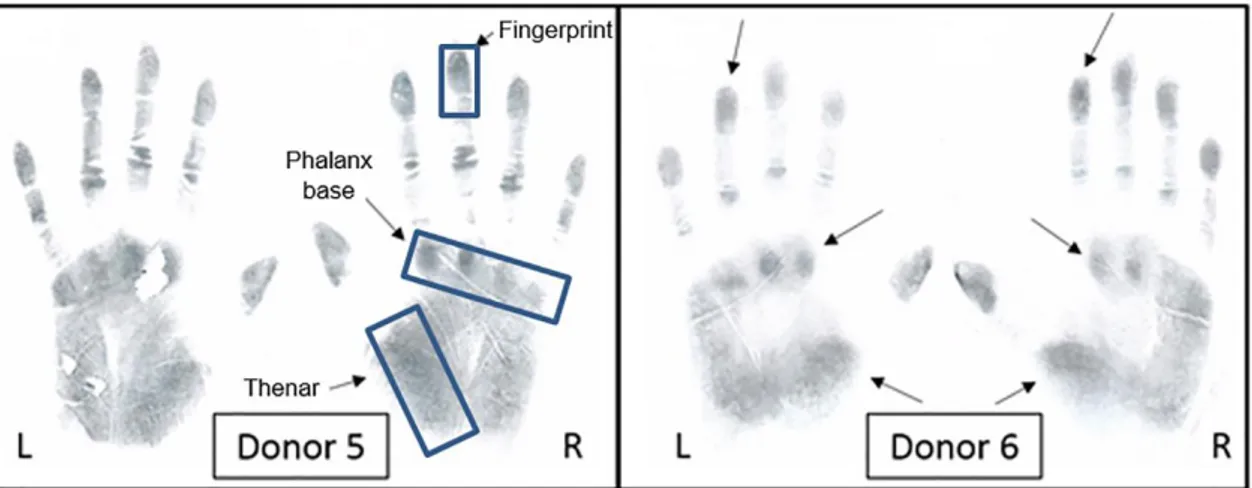
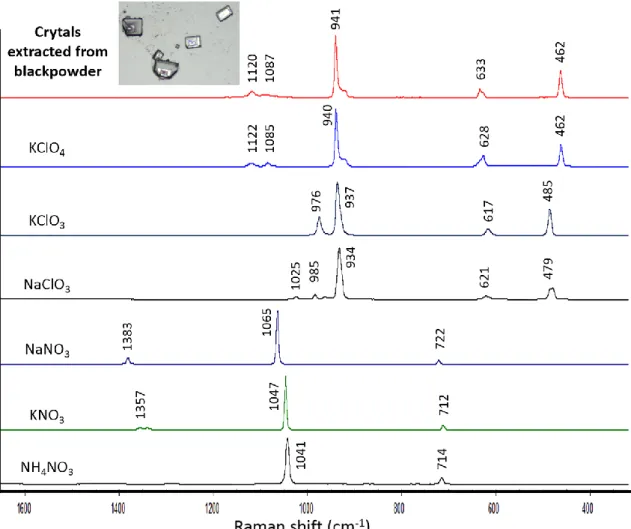
Figure

Documento similar
Four identification and quantification techniques, including liquid chromatography (LC) separation coupled to (i) a diode array ultraviolet (UV(DAD)) (ii), mass spectrometer in
High-performance liquid chromatography- ultraviolet detection method for the simultaneous determination of typical biogenic amines and precursor amino acids. applications in
In this work, a systematic experimental and theoretical analysis of the vapor-liquid equilibrium of {aromatic hydrocarbon (toluene) + ionic liquid} binary mixtures
Hernández, Multi-residue determination of 130 multiclass pesticides in fruits and vegetables by gas chromatography coupled to triple quadrupole tandem mass spectrometry,
Three sample treatment methods, based on QuEChERS, solid-phase extraction (SPE) and solid-phase microextraction (SPME), were compared and evaluated in order to obtain the
Suspect screening and target quantification of multi-class pharmaceuticals in surface water based on large-volume injection liquid chromatography and time-of-flight
This is due to include some suggestions that can not be considered as good analytical validation practise: low number of calibration levels (j = 4); narrow
The authors developed a simple and rapid method based on liquid chromatography in tandem with mass spectrometry (LC/MS-MS) with solid phase extraction (SPE)