Ph
ys
i
ca
l
C
h
em
istr
y
67 1-5667(>-%3.9/021$0.00+$L50
(Q?()(I? hvrRrPrP-<,:<;:T.Tr
Rates of diffusion-controlled reactions, chemical or enzymatic, have been suggested to be dependent on the physical state, whether a glassy solid or a highly concentrated liquid, of food systems. Studies on effects of glass transition,Tg, on such reactions as nonenzymatic browning, oxidation, and enzymatic changes have often provided controversial information of various effects of theTg,temperature, and water on the Abstract
Abstract .., , : , :..,...•...•....67
6.1 Introduction ., ,.· " ,..,.•68
6.2 Concentrated Systems and State Transitions ,<-, .••••..•...••••..•••.•••.•.•••.•. ,.69
6.2.1 Low-Moisture Foods ., , .,...•...., 70
6.2.2 Frozen Foods ,...•....70 6.2.3 State Diagrams ...•.73
6.3 Reaction Rate Controlling Factors in Foods 74
6.3.1 Temperature , , 74
;:~:~ ~::;~~~~~~::::::::::::::::::::::::::::::::::::::::::::::::::::::>:::::::::::::::::::::::::::
..~~
6.3.4 Phase and State Transitions , ,...,.76
6.4 State Transitions and Reaction Rates , 76
6.4.1 Glass Transition, Viscosity, and Diffusion 78
6.4.2 Glass Transition and Reaction Rates 80
6.5 Conclusions 83
References 83
CONTENTS
y.
H. Roas
S. M. Líevonen
State
Transitions
and
Reaction Rates in
Concentrated
Food
Systems
Baking, evaporation, dehydration, extrusion, and freezing are typical processes in theproduction ofconcentrated food systems. The resultant foods canbe amorphous 6.2 CONCENTRATEDSYSTEMS AND STAl!:
TRANSiTlONS
may, however, be sign.ificantly affected by water content .orthe amount ofunfrczea water,"?Oxidation may occur rapidly when water is removed,while ratesofojher reactions arereduced. Reactions occurring between water-soluble food components, such astheMaillard reaction and several enzymatic reactions in low-moisture foods,
have increasing rates aboye some critical water content.t+It has been suggested that reaction rates at normal storage temperatures of low-moisture foods are related to water activity, aw, defined astheratio of the equilibrium Watervapor pressure in the food, p, and the vapor pressure ofpure water at tbe same temperature, Po, i.e.,aw == plpo.l In some cases, ithas been found that the máximum stability is provided bya water activity corresponding to theBrunauer-Emmett-Telles (BET) monolayer valúe
of the food.'
Slade andLevine- have suggested the useofthe "polymer science approach" In evaluating food stability. Aceording to Slade and Levine,? water in foods acts as a plasticizer, depressing tbe glass transition of water-miscible food components such as carbohydrates and proteins. The glass transition is a well known property of
amorphous materials, whether inorganic or organ.ic. Glass transition occurs over a temperature range, which is ofien referred to with the glass transition temperature; Tg, taken as the onset or midpoint temperature of the transition determined usíng differential scanning calorimetry (DSC).2,5However, fue glass transition Can be observed from anumber of other changes ir¡material properties and relaxations
w
i
th
spectroscopic methods, such as FTIR, Raman, andNM
R
spectroscopies,and
other thermal analytical methods including dielectric analysis (DEA) and dynamie mechanical analysis (DMA).5-7DEA andDMA have beenfound tobemore sensitive in observing the glass transition than DSC, which applies especially tú thethermal characterization of frozen food materials."The food polymer science approach- has preved to be useful in explaining temperature- and water-content-dependent changes inreaction rates ofconcentrated food systems, such as low-moisture and frozen foods. LOW;plOisture and frozen foods contain amorphous, water-miscible solids, which can eiist ineither a glassy
orplasticized, amorphous, nonsolid, supercooled liquid state. Rélationships between tbe physical state, plasticization (either thermal or water), and rates of possíble diffusion-controlled reactions, suchasnonenzymatic brownmg, enzymatic reactions, and oxidation, have been reported in these nonequilibrium systems. However, the reaction rates have been found to be dependent a1soon other factors such as tem -perature, pll,and water content, and not only on the diffusivity ofreactants.v+' We will discuss state transitions of coneentrated, nonequilibrium food systems and effects of phase and state transitions and other factors on diffusion, reaction rates, and stability.
69 StateTransitions andReaction RatesinConcentrated FoodSysterns
Food systems represent alarge number of examples of nonequilibrium-state biom-aterials, which exhibit complicated time-dependent changes in tbeir physical and chem.ical properties during processing and prolonged storage.'> Food processing oftenaims atcontrolled exposure of food materials to,forexample, thermal, pressure, mechanical, or irradiative treatments. These, asa result ofsufficient energy input to the food material, cause a reduction in the number of active microorganisms or enzyme activity, changes in structure and texture, orchanges in chemical compos i-tion. These treatments are likely to increase tbe shelf life of foods or to improve their pleasantness and consumer appeal. However, foods are sensitive tohigh energy inputs, and adverse changes in sensory characteristics, such as texture, flavor, and taste, or nutritional value associated with processing may impair product quality.
Ma food eomponent, water often controls food temperature in processing and tbereby rates of changes in microbial or enzyme activity and chemical changes. Removal of water, however, results in concentration of food solids and increases in reaction rates. It is well known that, for example, decreasing water content in the bakíng or processíng of sugar- andprotein-containing foods results in browning and formation of desired, typical flavor and odor compounds (Maillard reaction) of the particular food ítem. Enhanced reaction ratesresulting from water removal, forexam -ple, in milk processing may also be extremely harmful, and only by proper control of time-ternperature-water content relationships are high-quality products obtained.
Dehydration and freezing prevent microbial growth and allow production of foods with extended shelf life. Enzyme activity, chemical reactions, and changes in texture are also retarded in low-moisture and frozen foods. Therates ofthese changes
INTRODUCTION
•
6.1
reaction rates. It is well known that rates of several reactions in low-moisture and frozen foods are signifieantly reduced, providing enhanced stability. However, both food systems showrapid changes in reaction rates aboye sorne critical water content or temperature. Inseveral cases, these critical parameters have been related to the Tg. Other changes, such as crystallization and structural transformations, may a1so occur in low-moisture and frozen foods, resulting in more severe changes in tbe rates of chemieal andenzymatic reactions. Forexample, crystallization of amorphous lactose has been shown to accelerate nonenzymatic browning in dairy powders or to affect the rates of enzymatic changes in food models. We, among others, have shown thatnonenzymatic browning may occur at temperarures below tbe Tgaltbough
at significantly Iower rates than aboye the calorimetric Tg• There is, however, a change in the reaction rate constant within the glass transition temperature range, Our recent results suggest tbat phase separation of food components may allow reactions tooccur below the Tg•In more homogeneous systems, an increase in the
reaction rate correlates with tbe endset of tbe glass transition. This inerease in the reaetion rate seems to eorrelate with observed increases in molecular mobility, as derived from dielectric measurements. Inbotb low-moisture and frozen systems, the pH, temperature, viscosity, and water content, in addition tothe glass transition, are Jikely to affect rates of physieochemieal changes.
The increase in the unfrozen water content decreases fue Tgof freeze-concentrated
solutes and viscosity and allows more rapid ice recrystallization.v" Ice dissolution
aboyeTm'may also result in increasing rates of diffusion-controlled reactions during storage of frozen foods." ",
Studies of state and phase transitions in frozen fóod systems have used ])S(;,
DEA, and DMA. A special disc-bending sample holder design was introduced
by
Maclnnes" for DMA studies of frozen solutions and foods. The sample holder consists of two plastic membranes, and the material studied can be prepared
art
dfrozen as a disc between the membranes. DSC, DEA, and DMA studies of frozen
foodsystems have shown thatthematerials suffer transitions dependent on theextent of freeze-concentration during initial freezing or dependent onthermal history. For example, rapidly cooled sugar solutions donot freeze completely even at very 19\\1
temperatures.i':" Such partially freeze-concentrated systems exhibit during rehe
at-ing a glass transition followed by devitrification and melting of ice (Figure 6;2).
Devitrification refers to ice formation during reheating and concurrent freeze GOn
-centration of the unfrozen solute phase. Maximally freeze-concentrated materíals
are formed when freezing time at atemperature favoring máximum ice formation
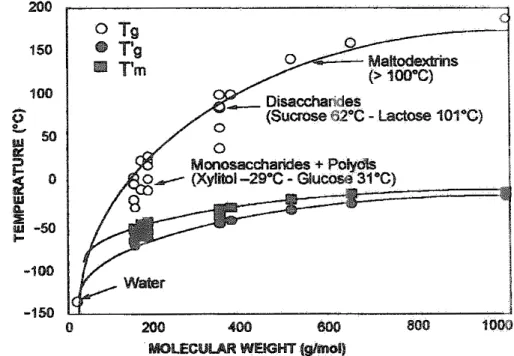
becomes sufficient forcomplete freezing. AHwater infood systems, however, canno; separate asice, because ice formation is controlled bythe viscosity of fueúnfto:z~ri solute phase. Roos and Karel" found thatsucrose solutions become inaximállyfreeze Figure 6.1 Glasstransition, Tg, and glasstransitionintht'<jnaximalty freeze-concentrated state,Tg', temperaturesandonset temperature oficemeltinginfue maxirnally freeze-concen -tratedstate,Tm', forselectedmonosaccharides,disaccharides, andmaltodextrins.
MOLECULAR WEIGHT (g/mol)
600
400200
o
-150 -100
200
O
Ts
150
•
Ts
•
Tm
100
-
o !!...SO
1&1
a::
5
OW Q.
:E
-50
w
o-5tateTransitions and Reaction RatesinConcentrated Pood$y~tems
State transitions offrozen food systems are important indefining their freeze-drying
behavior and storage stabílíty," For example, sugar solutions are known to have temperature limits in freeze-drying, referred to ascollapseremperatures.'? Collapse in freeze-drying Occurs when the freeze-eoncentrated unfrozen solute phase is able to ñowand cannot support the structure of the material, Collapse temperatures have
been found to correspond to the onset temperatures of ice melting, Tm', in the maximally freeze-concentrated state, probably because of arapid increase inunfro
-zen water content and concurrent plasticization of the unfrozen, amorphous phase,"
6.2.2 fROZEN FOQDS
The nonequilibrium, amorphous carbohydrates and sugars, such as lactose in dehy-drated dairy foods, sugars inhard candies, and starch have been found to suffer the glass transition and affect mechanicaI properties and stability of,forexample, fr
eeze-dried and spray-dried foods and confectioneries." The glass transition of low
-moisture foods is affected by cornposition, molecular weight of component com-pounds, -andextent of water plasticization.P The glass transition offoods with high
sugar content (for example, dehydrated berries and fruits and candies) is detected
with DSC from the change in heat capacity occurring over a temperature range of
10 to20t.14,15 The glass transition temperatures of sugars are affected bymolecular
weight, and they decrease dramatically with water content, Glass transition tempe r-atures for sugars typical offood systems areshown in Figure 6.1. The glass transition
of bread, which contains starch and gluten, has been observed at intermediate water contents." Foods containing large amounts ofhigh-molecular-weight carbohydrates
and proteins, however, have been found to exhibit glass transitions occurring over
awide temperature range with relatively low changes in heat capacíty.' The tran si-tions at low water contents occur at decomposition temperatures, and even in the presence of water, the transitions may bediffícult to observe. The determination of
such transitions with DSC often fails, and other techniques, e.g., DEA and DMA, must be used. Other factors complicating the analysis of low-moisture foods include
partial crystallinity of sugars or starch, poor rniscibility of carbohydrates and Pro-teins, and the presence of lipids.
6.2.1 low-MoISTURE FOODS
glasses or supercoóled Iiquids, or they mil)' be partially crystalline systems. Por
example, the branched starch polysaccharide, amylopectin, in baked products often exists as a partially crystalline polymer. In addition, foods may contain other com
-pounds in separate phases, such as water andlipid phases, or,inthe freeze-concen -trated state, the crystalline ice and unfrozen, freeze-concentrated amorphous phases. In some cases, the amorphous components areonly partially miscible, e.g., proteins and carbohydrates.'? Obviously, the complexity of foods, their nonequilibrium nature, and the various possible states and phase separation of component com -pounds make characterization of foods and measurements of their state transitions
difficult.
wbereTIiisglasstransitionoftheplasticized material,W1andW2areweight fractions
ofsolidsandwater,Tg1andTK2are glasstransitionsofsolids andwater,respectively, andk isaconstant.
Afullstatediagramshowsthewatercontentdependenceoftbeglasstransition temperature, typicaltransitions of the maximallyfreeze-concentrated solutes,and (6))
r
= _w..:.1_T_"'K.:.,l_+_k_W_2::..T-,~"",'2e W¡+kW2
6.2.3 STATEDIAGRAMS
The information ofphase and statetransitionscanbeshowninstate díagrams.?A state diagramcan beestablishedbymeasuringthe glasstransitionof a material at severalwater contentsand plottingthetransition temperatureagainst watercoIitent. Water plasticization, i.e., thedecrease in Tg with water content, can be modeled
usingfue Gordon-Taylor relationship of Equation (6.1) br other equations relating
theT,withwater content."
Figure 6.3Transitionsoccurringin frozenfoodcomponents,asobservedusing dífferential
scanningcalorimetry.
T
EMPERATURE
IAnneaHng~_'" ~
PartialiTeeze-concemration
Annealing
time
t
o
.JI/J"'" Inrual Tg
---~~ Unftozen~~
StateTransitions and Reaction RatesinConcentrated FoodSystems
concentratedduring annealingat-3SOC. The glass transition temperature of fre
eze-concentratedsolutions and foodsincreaseswith increasingicefonnation.Aconcur -rent increaseinsolute coneentration inthe unfrozen phase decreases theequilibrium ice meltingtemperature, Therefore,the deereasingice meltingtemperaturerequires that ice fonnation mustoecur at a lower temperature. However,tbeincreasingglass transitiontemperatureandviscosity of theunfrozensolutephase decreasestherate
of iceformation, Theoretieally,theTg' and Tm' coincideat a sufficientextent of freeze concentration,and theice formation ceases as theunfrozensolutephase is vitrified.?Sugarsolutionsoftenshowthe onset of theglasstransitionoí the maxi-mallyfreeze-concentrated unfrozensolutephase,Tg', andtheonsettemperature of
ice melting,
T
,
n'
,
in two separate transitions.Increasing molecular weightincreases these temperaturesand, for high-molecular-weight food components, thetransitions coíncide.PTypical transitionsoffrozenfoodcomponentsare shownin Figure6.3. DMA and DEA as well as NMR studies of frozen solutions have provided additional infonnation of relaxations and water mobility in freeze-concentrated systems.8•20,24TheDMAand.DEAstudies haveshown low-temperaturerelaxationsbelow theTg'. Thetemperatures of the Iow-temperature relaxations,as well asfue u-relaxarion (glass transition),are highly dependent onfrequency (Figure6.4).The lower frequencyc-relaxation temperatures «0.1 Hz) oftencorrespond tothe Tg' determined usingDSC. Thesemethodshavebeen successfulindetectingthe glass transitionof frozendoughs, whichcannot bemeasured usingDSC.8
Figure 6.2 Typicaltransitions of frozen foodmaterials during reheating afterrapidcooling, as observed usingdifferentialscanning calorimetry.Thedevitrification exothermindicates
partial freeze-concentrationduringfreezingandiceformation duríngrewarming.
TEMPERATIURE
DevitriflCation (ice.fonnation exotherm
)
I
Icemelting endQtherm
(afien accompanied by
an endothermalstepchange)
Glass transition ofunfmzen.soIutephase
1
Engineering and Food for the 21stCentury
Itshould benoticed that several other factors affectingreaction ratesmay alsobe dependent on temperature.Thismakes studies of the temperature dependence
o
t
where k
=
rateconstantko
=
pre-exponential factorEa=activation energy
R =gasconstant
T=absolute temperature
(6.2) Figure 6.S State diagram of sucroseshowingtheequilibrium ice melting temperature,Tm,
curve,onsettemperature of icemelting in the maximally freeze-concentrated state,Tm', glass
transition temperature of maximally freeze-concentratedsolutes,Tg', with solute concentration
givenby Cg', and theglasstransition,Tg,curveat various soluteconcentration.
'\;
temperaturehave been the QIO approach and the Arrhenius concept.26Inseveral
cases,fue reactíon rates have beenshowntofollowtheArrhenius relationshipgiven
inEquatíon (6.2).
CONCENTRA TION (%wlW
0.1
Salids)100
6
0
40
20
Cg
-120
Glass p~relaxal:ions
loe and glass Solution 80
60
40
.StateTransitions and Reaction RatesinConcentrated Food Systems
Temperature iscertaínly oneofthe most importantfactorsaffectingreaction rates in food systems. The main approaches in relating reaction kinetics in foods to
6.3.1 TEMPERATURE
Reaction rates in food materials can be controlled by
a
number of factors, as reviewed byLabuza and Riboh.> Themain factorsarefoodcomposition and the typeof(hereaction, temperature,pressure,watercontent,and pR. Inconcentrated food systems,theincreasingviscositymayreducediffusionand,asthematerials at low watercontents or at low temperatures vitrify,reactionrates may decrease dueto diffusionallimitations, and the temperature dependenceofthe reactionrates may deviatefrom Arrheniuskinetics." Although water seemstobefairlymobilein foodglasses,the mobility oflarger food componentmolecules seemto beretarded in the ~las~ystate." .
6.3 REA<::TlONRATE CONTROlUNG fACTO~S IN FOODS
equilibrium icemelting temperatures for thepartial1yfreeze-concentratedstates, as
shown in Figure6.5.22Thestate diagram may alsoshow other important transitions
or describerates of time-dependent changes atvarious temperature-watercontent
combinations.
Figure 6,4 Dielectricthermalanalysis ofa20%(w/w)sucrosesolutíonshowingfrequency -dependentji-relaxation and <X-relaxation(glasstransition).Theo-relaxation coincides with icemelting.
Engineering and Food forthe 21 st Century
74
3..0
2,5
<, 5Hz: 50Hz
2.0
7
HZ/\1'l .,. 'ijj
1.5
"O
c: ~
1.0.
0.5
Figure 6.7 Anexampleofrelationshipsamong wateractivity,watercontent, andviscosisy
aspredictedusingtheFenni and WLF relationships.
OJt::;....___. ...__¡_ __.._--L. _ _..::~-LlL...,....,...;.._,...L-..L-- ...
~O
O 0.2 0.4 0.6 0.8 1.0
WATER ACTIVl1Y 2
WLFmodel Fernii's model
CaIoril1letfiCTg at25·C
10
Glass Transition and Oiffusion
Diffusion in food systems has been related to viscosity and glass transition...Based
on this assumption, the use of the WLF relationship for predicting diffusion coe.ffi"
cients above glass transition has been suggested.? Stp:die~of diffusion in sucrose
solutions.P however, have shown that diffusion follows the WLF relationship well
aboye the T,but adecoupling between diffusion and viscosity occurs asthe glass
transition isapproached. Therefore, in thevicinity ofthe glass transition, diffusiyity cannot be predicted from viscosity."
Inpolymer scíence, the free-volume theory is used to describe concentration
and temperature dependency of solvents in amorphous polymers." Itis also known
that diffusion coefficients of small molecules arelittle áffected by concentration or
temperature. This agrees well with the findings that water remains fairly mobile in
glassy food systems.>' In binary systems, such asfoodsolids and water, the 111,4tl1al
diffusion coefficient islikely to bemost sensitive to concentration and t«mp~:rat:U.re
in the vicinity of the Tg. This can be observed from high activation eneJ,'giesof
The Fermi relationship has been found to fitdata ()i.1mechanical pf()perti~s of
foods; for example, 1088of crispness as a function QBvll,t~ractivity." It seems $at
the relationship isa1so applicable to predict viscosity ch¡lngesresulting fromthermal
or water plasticization over and aboye the glass transition (Figure 6.7).
7')
StateTransitionsand Reaction RatesinConcentrated FqóQ:5Y5tems
where y=a measure of mechanícaI property or "stiffness" parameter
~,=stiffness parameter in a reference state, e.g., in the glassy state
X
=
a measure of plasticization (temperature, water content, or water activity)X,=the value for
X
resulting in 50% change inya(X)=a measure ofthe steepness of the change in stiffness as afunction of X
1+
e
x
p
[~Z
~
]
~
(6,4)
1
where aTis the ratio of relaxation times, tand ts' attemperatures, Tand Ts'
respec-tively,
The WLF relationship states that changes in relaxation times above the glass
transition are related to
a
reference temperature, Ts' which along with the constants,el and C2,can be derived from experimental d4t;!..5.33Although theWLF relationship
may follow viscosity data aboye the glass transition, it assumes a steady viscosity
in the glassy state and downward concavity over the glass transition. Peleg,33
how-ever,has shown that the relationship between changes in mechanícal properties over
the glass transition shows an upward concavity and follows the Permi relationship
of Equation (6.4).
(63)
~C!(T-T.,)
C~+(T~ T,)
Parks etal." determined various changes occurring in glucose glasses as they were
transformed from the solid glassy state to the supercooled liquid state. Their work
íncluded determination of viscosity arnong severa1other material properties. Ithas
been suggested that the viscosity data followed the Williams-Landel-Ferry (WLF)
relationsñip" of Equation (6.3) and other equations relating viscosity changes in
amorphous materials totemperature.
Glass Transition And Viscosity
6.4.1 GlASS T~ANSIT.,ON,VISCOSJ'fY,ANO DlfFUSION
It'isgenerally accepted that the glass transition results in a change in the physical
stateof amorphous materiaIs, although exact glass transition temperarures cannot
be well defined, and the reported transition temperatures are dependent on the
methods and time ofobservatíon." The glass transition occurs over a temperature
range with aco~current dramatic change in viscosity. This change in viscosity results
in a decrease in relaxation times of structural transformations and apparent flow
aboye the transition. The changes in flow over and aboye the glass transition are
dependent on material properties; for example, sugar glasses become sticky syrups
not fal'from the glass transition, and materials containing high-molecular-weight
food polymers, such as low-moisture bread, become leathery or rubbery.
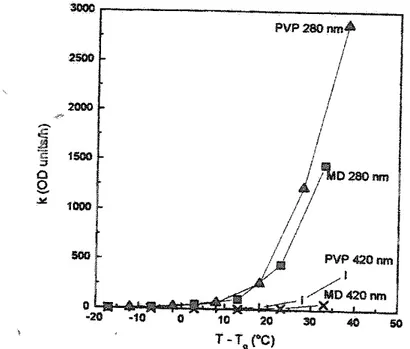
The Maillard reaction as a model of a binary reaction between an amino acid and a reducing sugar has probably been given the most attention in studies of relation-ships between reaction rates and the glass transition. The reaction is common in dehydrated foods and, in several cases, a relationship between the occurrence of the reaction with increasing temperature difference with storage temperature and the glass transition temperature,T - Tg,has been observed."
The effect of glass transition on the reaction rate has been related to observed discontinuities in Arrhenius plots, suggesting a high activatíon energyin the Vicinity of the transition.Itshould be noticed that the reaction also seems to occur belb"" the glass transition. This may result from heterogeneous distribution of reactams and water, phase separation of reactants from the main matrix, or the reaetant mobility may not cease at the measuredTgbut at sorne temperature lower than the
measuredTg•In sorne food models and real food systems, the reaction rate has not
increased in the immediate vicinity of the measuredT,but 20-40°C aboye the Tg,
depending on water content.38,39
Studies of effects of glass transition on reaction kinetics, and the MaiUard reaction in particular, are complicated because of several factors affecting plastici-zation and reaction rates. For example, plasticizers, i.e., water:content and telÚp~r-ature, affect reactant concentration, glass transition, pH, and water actiVity.
In a
study of Maillard reaction kinetics in amorphous maltodextrin andPYP systems, we controlled the amount of reactants, glucose, and lysine in the water phaseSOrbed
at33%RH and 25°C.38This study showed that the .reaction rate increased
consid-erably aboye the glass transition (Figure 6.8). The rates in the two systems, however, differed. This was not considered to result from differences inaw, reactant
concen-tration, orTg,as these parameters were comparable in the two systems. Microscopic
observation of the structure of the materials suggested that the reactants were phase separated in thePYP system, which probably resulted in more rapid browning in thePVP system.Inother studies usingPVP systems to observe Maillard reaction kinetics, the physical state has been found to be more important in controlling browning rate thana,...40,41
Other factors affecting reaction kinetics in low-moisture foods include collapse of structure and crystallization of component compounds. These factors have 'been found to enhance browningreactions_37,40,41Collapse also results in impaired diffusion, which is reflected by decreasing release of carbondioxide" and glysine consumption/'? Maillard Reaction
trations of sucrose, enzyme, and substrate, but, aboye freezing temperatures, the reaction rates were higher in less concentrated sucrose solutions.
We have conducted studies of enzymatic changes infrozen systems containing maltodextrins with differentTg' along with fish extracts containing the ™A()~ demethylation enzyme system, The reaction results in formation of formaldehyoe and toughening of frozen fish belonging to the cod family." We also used a reaction system with glycerol, which showed that the enzymatic reaction did not occur at
significant rates at temperatures below -25°C.
State Transitions and Reaction Ra.tes in Concentrated FoodSystems
Studies of glass transition effects on enzyrnatic changes have included sucrose inversion by invertase," rate of hydrolysis of disodium-p-nitrophenyl phosphate by alkalinephosphatase.P and enzymatic toughening of frozen fish.36
Sucrose inversion by invertase in low-moisture systems has been found to be dependent on water activity." The reaction rate increases at water activities aboye 0,6. We found, using a freeze-dried Iactose-sucrose system, that sucrose inversion occurred very slowly when the water content was not sufficient to depress theTgto helow storage temperature. In this system, the lactose crystallized aboye theTgand crystaIlization occurred concomitantly with increasing reactíon rateo Using of a maltodextrin-based system allowed observation of the reaction rate in a noncrystal-lizing system. However, the reaction rate was not found to increase significantly aboyeTgbelow water activity of 0.6, suggesting thataw was a more important factor
in controlling sucrose inversion than theTg. Itis possible that the enzyme mobility is not sufficient to allow the reaction to occur until the reacting molecules are plasticized by water to an appropriate extent aboye 0.6all'" Chen et aP5 found that sucrose hydrolysis in aPVPsystem started ataw0.62, and it was more affected by
awthan theTgof the system.
A study of the rate of hydrolysis of disodium-p-nitrophenyl phosphate by alka-Iine phosphatase in frozen sucrose solutions showed that the rate in the frozen state increased with dilution due to ice melting." However, the solutions did not become maximally freeze concentrated, and the effect of Tg' on the reaction rate could not be estimated. In the freeze-concentrated state, the solutions had the same
concen-Enzyrnatit Reactions
Glass transition has been suggested to affect reaction Tatesin both low-moisture and frozen foods. There are fairly few systematic studies of the temperature dependence of reaction rates in diffusion-controlled and "well stirred" food systems over the same temperature range. Another option is to follow reactions over the same tem-perature range in food systems having different glass transition temtem-peratures. Reac-tion rates in frozen foods are also affected by the extent of freeze concentraReac-tion, which, being a function of composition, makes comparison of reaction rates and possible effects of glass transition on observed rates difficult.
6.4.2 GLASS TRANSITION AND REAcnON RATES
díffusion in polymers as theTgis approached. Therefore, Arrhenius plots of diffusion
may suffer a step change over the glass transitíon, although the discontinuíty may not be perceptible for water." Similar diffusion behavior is also likely to occur in food systems.
It is obvious that diffusion occurs below the glass transition, but the mobility of solute rnolecules decreases significantly below theTg.24However, diffusion becomes more affected ~ the glass transitionwithincreasing size of the diffusing molecules. The size of diffusing reactants may, therefore, have an effect on whether a reaction may become diffusion controlled in the vicinity of the glass transition.
"
"
1
1
.
ii~
1
!
I
~
33
1. Labuza, T.P. 1968."Sorption Phenomena in Foods,' Food Teehnol.,22;263~265,
268,270,272.
2. Slade,L.and H.Levine. 1991. "BeyondWater Activity: Recent AdvancesBasedon
anAltemative Approach to the Assessment of Food Quality and Safery,"Crit. RiN.
Food Sei. Nutr.,30: 115-360.
3. Labuza, T.P., S.R.Tannenbaum,and M. Karel.1970."The EffectofWater Ac:tivity on Reaction KineticsofFood Deterioration," Food Technol.,24: 543~544,546--548,550. 4. Karel,M. 1985. "EffectsofWater Activity andWater Content on Mobílity inFQo4 Components, and Their Effect onPhase Transitions InFood Systems," inProperties
01
Water in Foods,D. Simatos and J. L.Multen, eds. Dordrecht, The Netherlands: Martinus Nijhoff Publishers, pp. 153-169.5. Roos, Y.H. 1995.Phase Transitionsin Foods.SanDiego, CA: Academic Press.
6. Sperling, L.H.1986. Introduetion to PhysiealPolymerScience.New York, NY:John
Wiley &Sonso
REfERENCES
Low-moisture and frozen foods are concentrated food systems exhibiting tempera"
ture- andwater-content-dépendentchanges in their physical state.These tháhg_es;, including glass transition and ice melting, affect diffusíon and, therefore, tates of
quality changes in these concentrated materials. Lów-moisture and frozen
fo
~
d.
s
show rapid changes inreaction rates aboye sorne critical watercontent~r t~mpera -ture. Although the critical values often coincide withplasticization .resulting m glass
transition, severa! studies have shown that diffusion affecting reaction tates and component crystallization, among other quality changes in fuese systems, is de~ en-dent on the molecular size of the díffusíng compounds, and that several reactions may occur in the glassy state. There ate, however, very little experimental. data available for establishingrelationships between kineticsof quality changes, phase
separatíon, temperarure, water content, and food tompositiOI).~
6.5 CONCLUSIONS
lactose crystallization andsubsequent release of the ené;tfpstilated Iipids. Thet~l~as~d Iipids become accessible by armospheric oxygen artd.rapid. oxidation.
In
s
órn
e
systems, crystallization of theencapsulating matrix isdelayed. !he dela~ed czystaJ.~
lization reduces oxygen penetration even when the encapsulatingmatnx suffersa
glass transition. ltis likely that the encapsulating matrix flow~.above the glass transition temperature, resulting in structural cóllápse. and partial release of. tl:c encapsulated lipids. There is,however, a possibility. that sorne ofthe lipids .remain encapsulated or become re-encapsulated in the matrix. . . .
Oxidation causes quality changes in low-rnoisture andfrozen food rnatenál~.
Unfortunately,there isvery little information abouttherélátionships betwe~n Ó.Xi~
dation kinetics and the physical state of low-moisture andfrozenfoods. ObVIOÚ~ly, such studies are of utmost importance, and they will be needed for product design and optimization of storage conditions.
StateTransítionsand Reaction Ratesin Concentrated FObdSysterns
j
r
Oxidatíon isa common reaction inIow-moisture and frozen foods. Itmaybe assumed that oxidatíon of lipids directIy exposed to atmospheric oxygen at food surfaces or surfaces of porous, dehydrated materials mayoccur freely. In both Iow-rnoisture and frozen foods, lipids exist phase separated frornthe water miscible solids,but Iipids may become encapsulated in glassy matrices that often improve stability of the
products.P
111Swell known that "free fat" in, for example, dairy powders, is highly
sus-ceptible tooxidation, causing qualitydefects. The stability of dairy powders isreIated
to the gIassy state oflactose, and the lipids are atIeast partially encapsulated within
the Iactose-protein matrix in spray-drying. The encapsulated fat is protected from
oxidation by the surrounding glassy lactose membranes. However, thermal or water plasticization resulting in the glass transition of the lactose-protein matrix allows
Oxidation
Because of the numerous factors affecting Maillard reaction kinetics in foods, the glass transition cannot be shown to have an individual effect onthe reaction rateo
However, the glass transition, along with material composition, water activity, tem -perature, andPIf,provides additional information forthecontrol of Maillard reaction
in food processíng and storage.
Figure 6.8 Maillard reaction rate constants as a function of the distance 01'a storage te
rn-perature añd fue glass transition temperature (T- Tg) for polyvinylpyrrolídone {PVP) and
rnaltodextrin (MD) models with Iysine and glucose as reactants.
PVP280.nm~
/
24. Hemminga, M. A.,l. J. van den Dries, P.C. M. M,Magusin, O.Van Dusschoten, and C. van den Berg. 1999. "Molecular Mobility in Food Components as Studied QY Magnetic Resonance Speetroscopy," inWater Management in the Design and Distri-bution of Quality Foods, Y.H. Roos, R. B. Leslie, and P. J.Lillford, eds, Lancaster, PA: Technornic Publishing Co., Ine., pp. 255-265.
25. Labuza, T.P. and D. Riboh. 1982. "Theory and Application of Arrhenius Kinetics to the Predictíon of Nutrient Losses in Foods," Food Technol., 36(10): 66, 68, 70, 72, 74. 26. Villota, R. and J. G. 1992. "Reaction Kinetics in Food Systems," in Handbook of Food Engineering; D. R. Heldman and D. B. Lund, eds. New York, NY: Maree! Dekker, pp. 39-144.
27. Knorr, D. 1998. "Advantages, Possibilities and Challenges of High Pressure Appli-cations in Food Processing," in The Properties ofWater in Foods ISOPOW 6, D.S.
Reid, ed. London: Blackie Aeademie &Professional, pp. 419-437.
28. Saltmarch, M., M. Vagnini-Ferrari, and T. P. Labuza. 1981. "Theoretical Basis and Application of Kinetics to Browning in Spray-Dried Whey Food Systems," Prog. FoodNutr: ScL, 5: 331-344.
29. LeMeste, M., D. Champion, G. Roudaut, E. Contreras-López, G. Blond, and D. Simatos. 1999. "Mobility and Reactívity in Low Moisture and Frozen Foods," in
Water Management in the Design and Distribution of Quality Foods,y.H. Roos, R. B. Leslie, and P. J. Lillford, eds. Lancaster, PA: Technornic Publishing Co., Inc., pp. 267-284.
30. Peleg, M. 1993. "Mapping the Stiffness-Temperature-Moisture Relationship of Solid Biomaterials at and around Their Glass Transition." Rheol. Acta, 32: 575-580. 31. Parks, G. S. and J. D. Reagh. 1937. "Studies on Glass. Xv. The Viscosity and Rigidlty
of Glucose Glass," 1.Chem. Phys., 5: 364-367.
32. Williams, M. L.,R.F.Landel, and J. D. Ferry. 1955. 'The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and other G1ass-Forming Liq-uids," J.Am. Chem. Soc.,77: 3701-3707.
33. Peleg, M. 1994. "A Model of Mechanical Changes inBiomaterials at and around Their Glass Transition," Biotechnol. Progr., 10: 385-388.
34. Duda, J. L.1999. "Theoretical Aspects ofMolecular Mobílity," inWater Management
in the Design. and Distribution of Quality Foods, Y.H. Roes, R. B. Leslie, and P.J.
Lil1ford, eds. Lancaster, PA: Technornic Publishing Co., Inc., pp. 237-253.
35. Chen, y. H., 1.L.Aull, and L.N. Bell. "Invertase Storage Stability and Sucrose Hydrolysis in Solids as Affected by Water Activíty and Glass Transition," J.Agric.
Food Chem., 47: 504-509.
36. Sallinen, J.and Y. H. Roos. 1998. "Freeze-Concentration and Glass Transition Effeets on Enzyme Kinetics in Frozen Food Models," inProceedings of the Poster Se ssions.
ISOPOW 7-Water Management in the Design and Distribution of Quality Foods, Y.
H. Roos, ed. Helsinki, Finland: University of Helsinki, EKT series 1143: 88-91. 37. Karmas, R., M. P. Buera, and M. Karel. 1992. "Effect of Glass Transition on Rates
of Nonenzymatic Browning in Food Systems," J.Agríe. Food Chem., 40: 873-879. 38. Lievone~, S. M., T. J.Laaksonen, and Y. H. Roos. 1998. "Glass Tmnsition and
Reaction Rates: Nonenzymatíc Browning in Glassy and Liquid Systems." J. Agríe,
Food Chem., 46: 2778-2784.
39. Karmas, R. and M. Karel. 1994. "TheEffect ofGlass Transition on MailIard Browning in Food Models," inMaillard Reactíons in Chemistry, Food, and Health, T. P. Labuza, G. A. Reineccius, V. Monnier, J. O'Brien and J.Baynes, eds. Cambridge, UK: The Royal Society of Chernistry, pp. 182-187.
85 State Iransítions and Reaction Rates in Concentrated FoodSysterns
7. Soderholm, S.E., Y. H. Roos, N. Meinander, and M. Hotokka, 1999. "Raman Spectra of Fructose and Glucose in the Amorphous and Crystalline States," J.Raman Spectr.,
30: 1009-1018.
8. Laaksonen, T.J.and Y.H.Roos. 1998. "Dielectric and Dynamic-Mechanical Prop-erties of Frozen Doughs," inProceedings of the Poster Sessions, ISOPOW 7-Water Management in the Design and Distribuuon of Quality Foods, Y. H. Roos, ed. Helsinki, gnland: The Universíty oí' Helsinki, EKT series 1143, pp. 42-45. 9. Lim, M. H. and D. S. Reíd. 1991. "Studies of Reaction Kinetics in Relation to the
Tg' of Polymers inFrozen Model Systems," in Water Relationships in Foods, H. Levine and L.Slade, eds, NewYork, NY: Plenum Press, pp. 103-122.
10. Nelson, K.A. and T. P. Labuza. 1994. "Water Activity and Food Polymer Scienee: Implications oí Stateon Arrhenius and WLF Models in Predicting Shelf Life," 1.
FoodEng., 22: 271~289.
11. Bell, L.N. 1996. "Kinetics oí' Non-Enzymatic Browning in Amorphous Solid Sys-tems: Distinguishing the Effeets of Water Activity and the Glass Transition," Food Res. lnt.,28: 591-597.
12. Kalichevsky, M. T., E. M. Jaroszkiewicz, and 1. M.V.Blanshard. 1993. "A Study of the Glass Transítion of Amylopectin-Sugar Mixtures," Polymer, 34: 346-358. 13. White, G. W. and S. H. Cakebread. 1966. "The Glassy State in Certaín
Sugar-Containing Food Products," J.Food Technol., 1: 73-82.
14. Roos, Y. H. 1987. "Effect of Moisture on the Thermal Behavior of Strawberries Studied Using Differential Scanning Calorimetry," J.Food Sci.,52: 146-149. 15. Roos, Y.H. 1993. "Meltíng and Glass Transitions oí' Low Molecular Weight
Carbo-hydrates," Carbohydr: Res., 238: 39-48.
16. Laine, M. J.K.and Y. Reos. 1994. "Water Plasticizatíon and Recrystallization of Starch in Relatíon to Glass Transition," inProceedings of the Poster Session, Inter-national Symposium on the Properties of Water, Practicum Il,A. Argaiz, A. López-Malo, E. Palou, and P. Corte, eds. Puebla, Mexico: Universidad de las Américas-Puebla, pp. 109-112.
17. Bellows, R. J.and C. J.King. 1973. "Product Collapse during Freeze Drying of Liquid Foods," AIChE Symp. Ser., 69(132): 33-41.
18. Hartel, R. W. 1998. "Mechanisms and Kinetics of Recrystallization in Ice Crearn," in The Properties of Water in Foods ISOPOW 6, D. S. Reíd, ed. London: Blackie Acadernic &Professiona1, pp. 287-319.
19. Karel, M. and 1.Saguy. 1991. "Effects of Water on Diffusion in Food Systems," in
Water Relationships in Foods, H. Levine andL.Slade, eds. New York, NY: Plenum Press, pp. 157-173.
20. MacInnes, W. M. 1993. "Dynamic Mechanical Thermal Analysis of Sucrose Solu-tions," in The Glassy State in Foods, J. M. V. Blanshard and P. J.Lillford, eds. Loughborough, UK: Nottingham University Press, pp. 223-248.
21. Simatos, D. and J. M. Turc. 1975. "Fundarnentals ofFreezing in Biological Systems," in Freeze Drying and Advanced Food Technology, S.A.Goldblith, L.Rey, and W. W. Rothmayr, eds, San Diego, CA: Academic Press, pp. 17-28.
22. Roos, Y. H. and M. Karel. 1991. "Amorphous State and Delayed Ice Formatíon in Sucrose Solutions:' Int. J. Food Sci. Technol., 26: 553-566.
23. Roos, Y. H. and M. Karel. 1991. "Water and Molecular Weight Eflects on Glass Transitions in Amorphous Carbohydrates and Carbohydrate Solutions," J.Food Sci.,
56: 1676-1681.