Electrochemical coupling reactions of benzyl halides on a powder cathode and cavity cell
Texto completo
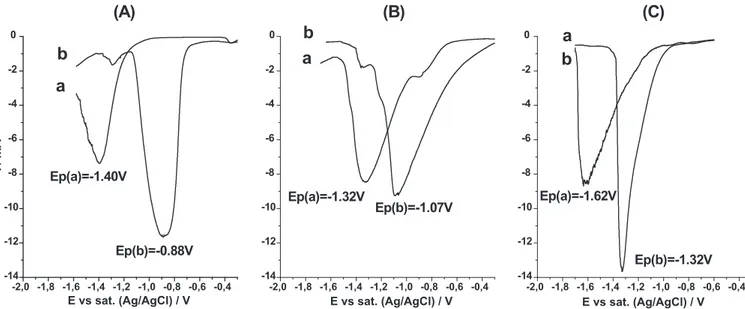
Figure
Documento similar
For instance, (i) in finite unified theories the universality predicts that the lightest supersymmetric particle is a charged particle, namely the superpartner of the τ -lepton,
Díaz Soto has raised the point about banning religious garb in the ―public space.‖ He states, ―for example, in most Spanish public Universities, there is a Catholic chapel
Although Asian American critics have historically shunned any literary model that examines an Asian past, calling instead for a sustained critique of American
teriza por dos factores, que vienen a determinar la especial responsabilidad que incumbe al Tribunal de Justicia en esta materia: de un lado, la inexistencia, en el
In this work, we have undertaken the study of SUZ12, a Polycomb group protein and the microRNAs (miRNA) expressed by the oncogenic Epstein Barr Virus (EBV) in
In the “big picture” perspective of the recent years that we have described in Brazil, Spain, Portugal and Puerto Rico there are some similarities and important differences,
For instance, the best overall accuracy of bagging in Breast with 20% noise is achieved using a 10% sampling ratio: The test error goes from 4.1% when no noise is injected to 3.5%
In particular, different versions of the training set for the base learners can be used, as in bagging (bootstrap sampling of training data), class-switching (noise injection in