www.elsevier.es/rmuanl
REVIEW
ARTICLE
Models
of
hepatoprotective
activity
assessment
C.
Delgado-Montemayor
a,
P.
Cordero-Pérez
b,
R.
Salazar-Aranda
a,
N.
Waksman-Minsky
a,∗aDepartmentofAnalyticalChemistry,MedicalSchool,AutonomousUniversityofNuevoLeon,Monterrey,Mexico
bLiverUnitatthe‘‘Dr.JoséE.González’’UniversityHospitaloftheAutonomousUniversityofNuevoLeon,
Monterrey,Mexico
Received16January2015;accepted30October2015 Availableonline23April2016
KEYWORDS
Hepatoprotection; Invitromodels; Invivomodels; Exvivomodels; Liver;
Hepatocuration
Abstract Liverdiseasesareamajorhealthproblemworldwide,makingitnecessarytodevelop newmoleculesthathelpcounteractorpreventsuchdiseases.Onaccountofthisfact, inves-tigations aimingto obtainnatural and/or syntheticcompounds possessing hepatoprotective activityhavebeenundertaken.Thedevelopmentofnewdrugsconsistsofavarietyofsteps, rangingfromthediscoveryofthepharmacologicaleffectsincellularandanimalmodels,to finallydemonstratetheir efficacyandsafetyinhumans.Differentmodelsfor assessmentof thehepatoprotectiveactivityinvitro,exvivoandinvivocanbefoundinmedicalliterature. Thepurposeofthisreviewistoshowthefeatures,mainadvantagesanddisadvantagesofeach ofthemodels,thehepatotoxicagentsmostcommonlyused(CCl4,acetaminophen,ethanol,d
-galactosamine,t-BuOOH,thioacetamide)aswellasthebiochemicalparametersusefultoassess liverdamageinthedifferentmodels.
©2016UniversidadAut´onomadeNuevoLe´on.PublishedbyMassonDoymaM´exicoS.A.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/ by-nc-nd/4.0/).
Abbreviations:DNA,deoxyribonucleicacid;ALT,alanine amino-transferase; RNA, ribonucleic acid; AST, aspartate aminotrans-ferase;CCl3, trichloromethylradical;CCl4,carbontetrachloride;
ALP,alkalinephosphatase;GGT,gamma-glutamyl-transpeptidase; GSH, reduced glutathione; GSSG, glutathione disulfide; NF-B, nuclearfactorkappabeta;LDH,lactatedeshydrogenase;NADPH, nicotinamideadenine dinucleotidephosphate;NAPQUI, n-acetyl-benzoquinoneimine; WHO, World Health Organization; PCTS, precision-cuttissueslices;t-BuOOH,tert-Butylhydroperoxide.
∗Correspondingauthorat:DepartamentoQuímicaAnalítica,
Fac-ultaddeMedicinaUANL,Av.MaderoyAguirrePeque˜noS/N,Col. MitrasCentro,CP.64460Monterrey,NuevoLeón,Mexico.
E-mailaddress:nwaksman@gmail.com(N.Waksman-Minsky).
Introduction
The liver is a key organ. It regulates different functions in the body, such as metabolism, secretion, storage and detoxifying.Liverdamageisusuallyassociatedwiththe dis-tortionofsomeofthesefunctions.Theliveriscontinuously exposed to an elevated amount of toxic agents, because theportalveinsuppliesbloodtothisorganafterintestinal absorption.1,2
TheWorld HealthOrganization (WHO)determined that around 2.4 million deaths yearlyare linkedto some liver disease,andthataround800thousandofthesedeathsare attributabletocirrhosis.3Ontheotherhand,
epidemiologi-calstudiesconductedbytheNationalInstituteofStatistics
http://dx.doi.org/10.1016/j.rmu.2015.10.002
Oxidative stress
Oxidative damage in DNA and proteins
↓ ATP
ROS
H2O2
HO• •
O2 –
↑ Lipid peroxidation
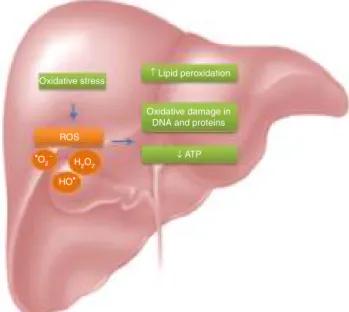
Figure1 Mechanismofliverdamageduetooxidativestress.
and Geography (INEGI by its Spanish acronym) indicate that in 2013 in Mexico, over 600 thousand deaths were recorded.Themaincauseswerediabetesmellitus(14.25%), followedbyischemicheartdiseases(12.63%), cerebrovascu-lardiseases(5.29%)andliverdiseases(4.79%).Despitethe advancesinmodernmedicineandthedevelopmentofnew hepatoprotectivedrugs,4---6theincidenceofhepaticdiseases
has not decreased or stopped; onthe contrary, statistics suggestthatthesecontinuetoincrease.7,8
Metabolism or biotransformation of hepatotoxicagents is a detoxifying process where molecules are surgically modifiedintolesstoxicshapesbydifferentenzymatic sys-tems.Thesemodificationscangeneratemetabolicproducts withvaryingdegreesofpharmacologicalactivityorinactive metabolites. Therearedifferent typesof metabolic reac-tions:phase1reactionsareusuallyoxidations,reductionsor hydrolysis(modifyingthestructureofthereactive group); phase2 reactions arethose inwhich the drugconjugates withglucuronicacid,sulfates,acetates,methylgroups, glu-tathioneoraminoacids,generallytoincreaseitssolubility andbeexcreted.Theliver’sabilitytobeabletocarryout thedifferentoxidativemetabolisms isassociatedwiththe highcytochromeP450cellcontent.9
Duetothehighmetabolitebiotransformationrate,free radicals can be generated continuously. Most hepatotoxic substances, mainly damage the liverbecause of the gen-eratedoxidativestress;oxygenreactive speciesinduced a riseinlipidperoxidation,areductionofATPandoxidative damageintheDNAandproteins(Fig.1).10---13
Protecting the liver from the harmful effects of hepatotoxins-whichmaybeingested-orcounteractingthe alterationsintheantirradicaldefensemechanisms,isvery important; the agents capable of doing this are called hepatoprotective.14
For this reason, researches have been developed in the search of natural and/or synthetic compounds withhepatoprotective activity.8 The development of new
pharmaceuticalsconsistsof avariety ofsteps, goingfrom thediscoveryofpharmacologicalsideeffectsincellularand
animal models, to finally prove its efficacy and safety in humanbeings.1
In vivo and well as ex vivo test models are used to evaluate hepatoprotective activity. These systems mea-sure the ability of the drug to prevent or cure hepatic toxicity(inducedbydifferenthepatotoxins)incellular cul-tures,organsorinexperimentalanimals(rats,mice,etc.) respectively.1
Evaluation
models
Nearlyeveryacute andchronic liverinjury can be exper-imentally induced; necrosis, steatosis, hepatic injuries, cirrhosisandcholestasis.Thesecanallbegeneratedin dif-ferentmodelsofliverdamage.
Theobjectiveofhepatoprotectivemodelsisforthe com-pounds, fractions or extracts being tested to counteract oravoidthedamagegeneratedbyhepatotoxins.The mag-nitude of the hepatoprotective effect can be measured throughbiochemical makers, survival rate or histology of theliver.
Testmethodsmaybeinvitro,exvivoorinvivo(Table1); andeach oneofthemcan beevaluatedtoseeifthe sub-stanceishepatoprotectiveorhepatocurative,dependingon ifthehepatoprotectiveagentisadministeredbeforeorafter thehepatotoxin.
Invitromodels
Freshhepatocytes,primaryhepatocyteculturesand immor-talizedcelllinesareusedtomeasurethehepatoprotective effect.Itispossibletoestablishactionmechanismsinthese models. These models represent the best option for the screeningandselectionofpotentialhepatoprotective com-poundsanditispossibletoestablishactionmechanismsat acellularandmolecularlevel.15,16
Primary hepatocytecultureshave thecharacteristic of maintainingnormalmetabolicliverproperties,butitisnot possibletomaintainthemforalongtime.Ontheotherhand, celllines maintaintheir properties stablefor a long time andcanbecryopreserved,butimmortalizedorcarcinogenic linesmaydifferinbiochemicalandmetabolicaspectsfrom normalcells.1
In order to evaluate protection, parameters like transaminase liberation, cell multiplication, morphology, macromolecular synthesis, oxygen consumption, etc., are measured.17,18
Advantagesofinvitromodelsare:Theyarequicktests (between2---3testingdays),theyrequiresmallamountsof thetest substances (milligramrange)and the experimen-talconditionsmaybestrictlycontrolled;differentsamples maybeanalyzedinthesametest,theyarecheaptestsand thereis littlevariability; therefore theyareconsidered a reproducibletest. Inthecase ofprimaryculturesor fresh hepatocytes,theyrequirefewexperimentalanimalsin com-parisontoinvivomodels.
Table1 Modelsofevaluationsofhepatoprotectiveactivity.
Model Examples Advantages Disadvatages
Invitro *Freshhepatocytes *Primaryhepatocyte culture
*Immortalizedcelllines (HepG2,HUH7,HepRG)
*Quickandcheaptests *Requiresfewsamples *Highcontrolof variables;reproducible *Cananalysesvarious samplesinthesametest
*Duetoalackofcomplexitypresent intheorganofbiologicalsystem, resultsshouldbeinterpretedwith caution.
*Samplesdonotundergoany biotransformationprocess
Exvivo *Preciselivercuts
*Isolatedperfusedliver
*Resembletheinvivo environment
*Decreasethenumber ofanimalsexperimented on
*Ahumantissuemodel canbedeveloped
*Lowoxygenationrateinthe internalcells
*Lowcutviability(1---10días) *Therearesignificantdifferencesin sizeandfuctionbetweenhumanand murinetissue.
Invivo *Murinemodel *Widelyused
*Thereisagreater correlationwithwhat happensinhumans *Allbiochemicaland histologicalparameters canbemeasured
*Requiresalargenumberofanimals toexperimenton
*Interindividualvariationexists *Alargersimplesizeisrequired *Largeandexpensiveexperiments
beverified within vivosystems. Isolatedcells as well as celllineshavean elevatedcelldifferentiationratedueto thelossofnaturalenvironment.Thesubstancestested do notgo throughthe absorption anddistribution processes, whichoccursintheorganism.Thereislittletono cell-to-cellinteraction and thereis no complexity proper of the organ.19---21
Exvivomodels
Precisioncutliverslices(PCLS)areanexvivotissueculture which imitates multicellular characteristics of in vivo
organs.Cellularinteractionand spatialdisposition remain intactinthismodel,withthepossibilityofperforming mor-phologicalstudies. Liver slices have the characteristic of functionallymaintainingmetabolizingenzymesandbiliary canaliculus21; theyhave proven tobe avalid ex vivo
sys-temtostudymetabolismandliverdamageandfunctionas abridgebetweeninvivosystemsandcellcultures.22
Isolated perfused livers represent a model combining
in vitro characteristics under in vivo circumstances. The
firstmodelwasdevelopedinporcineliversandlaterthe liv-ersofsmalleranimals(rats,miceandrabbits).Thismodel preservesthetridimensionalstructureaswellasthe cell-to-cellinteractionswiththepossibilityofcollectingbileinreal time.Ifbloodisusedasaperfusorliquid,thenhemodynamic parametersmaybestudied.23
Advantages of ex vivo models: they resemble in vivo
atmospheres,arelowcost,reproduciblemodels.InPCLSthe numberofexperimentalanimalsisreduced,alsothemodel canbedevelopedwithhumanorgans.
Disadvantagesof exvivomodels:in PCLSthe bileflow andfunctionalparameters,suchasportalflow,cannotbe analyzed.1 There is poor diffusion of oxygen nutrients to
themoreinternalcells,andevenwiththedevelopmentof new means of culture, the viabilityof the slicesremains short (8---10 Days).22 In small labs, because of space and
budget,thebestoptionisthedevelopmentofperfusedrat liver;however,therearesignificantdifferencesinthesize, functionandgeometryofthemurinelivercomparedtothe human.1
Invivomodel
This model hasbeen widely used;through thismodel we areabletodeterminetheprotectionmechanism.The dam-ageproducedinexperimentalanimalsduetoknowndosage administration of different hepatotoxins and the magni-tude of the damage and/or protection is determined by the differentbiochemicaland metabolic markers,as well ashistopathologicaldeterminations.
Advantagesofinvivomodels:isthemodelwiththe high-estdegreeofcorrelationwithwhatoccursinhumansandall biochemicalandhistopathologicalparameterscanbe mea-sured.Theyletustakeintoaccountthepossibleeffectsof theimmuneandcentralnervoussystemsinthedevelopment ofhepaticdiseases.24
Hepatotoxic
agents
and
their
action
mechanisms
Themoleculesresponsibleforliverdamagearecalled hep-atotoxins; nowadays it is possible to imitate any formof natural-originhepaticdiseasewithdifferentchemical sub-stancesandpharmaceuticals.
Hepatotoxinsmaybeclassifiedasintrinsiciftheagent’s behavior is predictable; there is a period of constant latencybetweenexposureandliverdamagedevelopment, or theinjury is dose-dependent(i.e. carbontetrachloride
{CCl4},thioacetamide, acetaminophen, ethanol). Another
classification is idiosyncratic, if the agents are not pre-dictable,butgenerateliverdamageinjustasmallportion of exposed individuals, the injury is not related to the dosage, it occurs after a variable latent period and it is notreproducible in experimentalanimals (i.e. halothane, sulfonamides,isoniazid).26,27
Carbontetrachloride(CCl4)
CCl4toxicitydependsondosageandthedurationof
expo-sure.Inlowdose,effectslikelossofCa2+homeostasis,lipid
peroxidation, andrelease of cytokinesare produced, and apoptotic events may be generated, followed by cellular regeneration.Inhighdoses,orifthereisalongerexposure, theeffectsaremoresevereandthedamageoccursduring alongerperiodof time,the patientmay developfibrosis, cirrhosis,orevencancer.5,28,29
CCl4ismetabolizedbythecytochromeP450dependentof
monooxygenases,mainlythroughtheCYP2E1isoforminthe endoplasmicreticulumandmitochondria.16 Hepatotoxicity
isproducedbytheformationofthetrichloromethylradical (CCl3), which is highly reactive. These radicals may
satu-ratetheorganism’santioxidantdefensesystem,reactwith proteins, attack unsaturated fatty acids, generating lipid peroxidation,reducetheamountofcytochromeP450,which leadstoafunctionalfailurewiththeconsequentloweringof proteinandaccumulationoftriglycerides(fattyliver),and alterwaterandelectrolyteequilibriumwithanincreaseof hepaticenzymesinplasma.30
Lipidperoxidationleadstoacascadeofreactions,such asthe destruction of membrane lipids, the generationof endogenoustoxicsubstances,whichoriginatemorehepatic complications and functional anomalies. For this reason, lipidperoxidationisconsideredacriticalfactorinthe patho-genesisofliverinjuriesinducedbyCCl4.15Theinhibitionof
the radical CCl3 generation is a key point in the
protec-tion against the damage generated. Because of this, this modeliswidelyusedfortheevaluationofpharmaceuticals andnaturalproductswithhepatoprotectiveandantioxidant activity.31,32
Acetaminophen
It is an analgesic antipyretic analgesic. In high doses, it producesacuteliverdamage,causingnecrosisofthe hepa-tocytes.Itisawidelyusedexperimentalmodelofclinical importanceasanexampleofdrug-inducedliverdamage.16
At therapeutic doses, it is mainly metabolized to glu-curonic or sulfated and excreted derivatives, the rest metabolizes to intermediate reactives, which are elimi-natedby conjugation withglutathione. At overdoses, the excess is oxidized by the cytochrome P450 (mainly the CYP2E1 isoform)33 at N-acetyl-p-benzoquinone (NAPQI),
whichquicklyattachestoglutathione.Underexcessive con-ditionsofNAPQIandglutathionedepletion,acovalentbond ofmetabolitetoproteins,adductformation,mitochondrial dysfunctionandoxidativestressoccurs.Theresultis necro-sisorhepatocellulardeath.19,34
Ethanol
Theliveristhemostsusceptibleorgantothetoxiceffectsof ethanol.Thedamagemechanismisduetothemetabolism of ethanol by the CYP2E1 isoform of the cytochrome P450 producing oxidative stress with the generation of reactive species of oxygen and the increase of lipid per-oxidation, leading to the alteration of the compositions ofphospholipidsof thecellularmembrane.35,36 Membrane
lipid peroxidation results in the loss of its structure and integrity,elevatingserumlevelsofglutamyl-transpeptidase, amembrane-bondingenzyme.Ethanol inhibitsglutathione peroxidase;itreducestheactivityofcatalaseanddismutase superoxide.16
Thedecreaseintheactivityofantioxidantenzymes, dis-mutasesuperoxideandperoxidaseglutathioneisbelievedto comeasaresultoftheharmfuleffectsoffreeradicals pro-ducedafterexposuretoethanol,oralternatively,theycould bea directeffect of acetaldehyde, a product of ethanol oxidation.
D-Galactosamine
Thishepatotoxingeneratesasimilardamagetoviral hepati-tisregardingmorphologicandfunctionalcharacteristics.A singledosecancausehepatocellularnecrosisandfattyliver. Itinducestheexhaustionoftheuracilnucleotide, result-ing in the inhibition of RNA synthesis and consequently of proteins.37 The toxicity mechanism causes loss of the
activity of ion pumps and an increase in cellular mem-brane permeability, leading to enzyme liberation and an increasein intracellular Ca2+ concentration, whichis
con-sideredresponsibleforcellulardeath.16,36,38
Tert-Butylhydroperoxide(t-BuOOH)
Metabolized to free radicals by cytochrome P450 in hepatocytes generating lipid peroxidation, a decrease of glutathione,itreduces thepotentialof themitochondrial membraneandcellulardamage;generateddamageis simi-lartooxidativestress,whichoccursincellsandtissues.36,39
Alternatively,t-BuOOHcanbeconvertedbyglutathione peroxidaseintotetr-butylalcoholandglutathionedisulfide (GSSG).GSSGisconvertedintoreducedglutathione(GSH) bytheGSSGreductase,generatingtheoxidationofpyridine nucleotides(NAPD).Alltheseeventsalterthehomeostasis ofCa+2whichisconsideredacriticaleventtoprovide
Thioacetamide
Anorganiccompoundcontainingsulfur,originallyusedasa fungicideandcurrentlyusedforthetreatmentofleather,in labsandinthetextileandpaperindustries.29Itcaninduce
acuteandchronichepaticinjuriesandactsoverthe synthe-sisofprotein,DNA,RNAandover␥-glutamyltranspeptidase
(GGT)activity.
Thioacetamideisbio-activatedbytheCYP450and/orby themonooxigenasesystem,whichcontainsflavin, convert-ingthecompoundintosulfine(asulfoxide-typecompound) andlaterintosulfone-typecompounds.Sulfineisresponsible for generating an increase in the nucleus volume, nucle-olienlargement,anincreaseinintracellularconcentration of Ca+2, generating changes in cellular permeability and
mitochondrialdysfunction.Ontheotherhand,Sulfone-type compoundsareresponsiblefortheliberationofnitricoxide synthaseand the nuclearfactor kappa B(NF-B), protein
denaturalizationandlipidperoxidation.41---43
Liver
function
markers
A decisive step when biological activity models are per-formedistheanalysisoftheactivityofthetestedanalyte. Depending on the selected model and its characteristics, thesurvivalrateandthedamagedbiochemicalmarkerscan bedetermined.Due tothe widevarietyof functions per-formedbytheliver,thereisawiderangeofmarkersthrough whichweareabletodeterminethefunctionalityordamage generatedbythisorganoritscells.28 Althoughthereisno
biochemicalmarkerspecifictoliverdamage,the combina-tionof severalofthese,andknowingthecorrelation they havewiththeliver,willhelptobetterinterprettheresults ofthehepatoprotectivemodels.Markerscanbedividedinto tests related to the liver’s excretory function (bilirubin), testsrelatedtosyntheticfunction(albuminand prothrom-bintime)andtestsrelatedtotheintegrityofhepatocytes (transaminases,alkalinephosphatase,GGT).
Transaminasesoraminotransferases
Transaminasesoraminotransferasesareenzymesthat trans-fer a group of amino from an amino acid to an acid
␣-acetate. This process is an important step in the
metabolism of amino acids. The aspartate aminotrans-ferase (AST) and the alanine aminotransferase (ALT) are widely used enzymes; the increase in the liberation of thesetransaminasesislinkedtoliverdysfunction.ALT cat-alyzes the amino group transference of the L-alanine to
␣-ketoglutaratetoproducepyruvateandL-glutamate;itis
elevatedin hepaticand renaldiseases, i.e.hepatitis, cir-rhosisand mononucleosis. AST catalyzes the transference oftheaminogroupoftheL-aspartateto␣-ketoglutarateto
produceoxaloacetateandL-glutamate;theheart,liverand skeletalmuscle,areorgansrichwiththisenzymeandthe ASTliberationisproportionaltothedamagegenerated.Ina myocardialinfarctionitstartstoincreasebetween3and9h aftertheevent,reachingitspeakonthesecond day;the levelsnormalizebetweenthe fourthand thesixthday. In hepatitiscases,observedelevationsarebetween7and12
timesitsnormalconcentrations,withincreasesofupto100 times.28
Phosphatases
These enzymes belong to the hydrolases family and are known for their ability to hydrolyze a wide variety of organophosphate compounds with the formation of phos-phateionsandalcohols.Clinicallyrelevantphosphatasesare acidphosphataseandalkaline phosphatase.Alkaline phos-phatase(ALP)isproducedmainlyintheliverandbone;when there arenoosteogenic diseases, ALP elevation is gener-allylinkedtohepatobiliarydiseases. Itis morespecific in obstructivehepaticprocesses.28,44
Transpeptidase␥-glutamine(GGT)
This enzyme is boundto the plasmatic membrane, which catalyzes the transference of the ␥-glutamine group of a
peptide toitselforother peptides.Itis locatedmainly in hepatocytes;howeveritcanalsobefoundintheproximal renal tubules, intestinalepithelial cells and theprostate. High GGTP levels usually indicate infection in the liver, pancreaticand biliaryzones.The specificityof thetest is relativelylow,butsinceitisnotlinkedtobonediseases,it isusedtolinkhighALPlevelstoliverdamage.44
Bilirubin
Bilirubin is the most important metabolite of the heme group, found in hemoglobin, myoglobinand cytochromes. Itishighlyinsolubleinwaterinitsmostcommonisomeric form,and most ofit is transportedby albumin. The liver is responsible for eliminating bilirubin by turning it to a morehydrosolubecompound,thusallowingitselimination ofplasmaforitseventualexcretion.Itisthemostimportant test ofthehepaticmetabolicfunction;however,itis only possibletodetermineitininvivomodels.44
Totalproteins
Theliversynthetizesmostplasmaticproteins,andinmost hepaticdiseasesthelevelsarereduced.Albumin,␣-1
antit-rypsin,ceruloplasmin,and␣-fetoproteinareproteinslinked
toacuteliverdamage.
Lactatodeshydrogenase(LDH)
Lactatodeshydrogenaseisanenzymelocatedinthecellular cytoplasm. Itcatalyzes the interconversion ofthe lactate and pyruvate; LDH liberation may be interpreted as the opening of the cellularmembrane or cellular death. This enzyme is not specific to the liver and it is widely used
inin vitromodelsbecauseitis expressedinmostcellular
lines.45
AST, ALT and ALP are most commonly analyzed in all hepatoprotectivemodels,whilethequantificationoftotal proteinsandLDHaregenerallyusedasparametersofinvitro
Conclusions
Liver diseases are a major health problem, domestically and around the world; thus, it is necessary to develop newmoleculeswhichhelpcounteractorpreventthem.The discovery and development of new drugs begins with the demonstrationofthepharmacologicaleffects,tolater con-ductsafetyand efficacystudies in humanbeings. Invitro
modelsarewidelyused;theyarefast,cheap,reproducible techniques andrequirealowersample. Nevertheless, the results ought tobe reevaluated by other models.Ex vivo
models are an intermediate point between in vivo and
invitromodels,butarelessutilized.Unliketheothertwo,
in vivomodelsprovidea widerangeof information.They arewidely usedtoverify theactivity of newcompounds, although they are more expensive and go through many experimentalanimals.Hence,theyaregenerallyusedafter
aninvitroorexvivoevaluation,asastepprevioustoclinical
trials.
Conflict
of
interest
Theauthorshavenoconflictsofinteresttodeclare.
Funding
Nofinancialsupportwasprovided.
Acknowledgements
SpecialthanksgototheMexicanNationalCouncilofScience andTechnology(abbreviatedCONACYT)fortheirsupportfor thenationalscholarshipno.359832andprojectno.180997 oftheBasicScientificResearchGrant2012.
References
1.GronebergDA,Grosse-SiestrupC,FischerA.Invitromodelsto studyhepatotoxicity.ToxicolPathol.2002;30:394---9.
2.Vargas-MendozaN, Madrigal-SantillánE, Morales-GonzálezA, etal.Hepatoprotectiveeffectofsilymarin.WorldJHepatol. 2014;6:144---9,http://dx.doi.org/10.4254/wjh.v6.i3.144. 3.Organización Mundial de la Salud. OMS-cirrosis; 2014
www.who.int/en
4.Waring WS. Novel acetylcysteine regimens for treatment of paracetamol overdose. Ther Adv Drug Saf. 2012:305---15, http://dx.doi.org/10.1177/2042098612464265.
5.Zhao XY, Zeng X, Li XM, et al. Pirfenidone inhibits carbon tetrachloride- and albumin complex-induced liver fibrosis in rodentsbypreventingactivationofhepaticstellatecells.Clin ExpPharmacolPhysiol.2009;36(10):963---8,http://dx.doi.org/ 10.1111/j.1440-1681.2009.05194.x.
6.Macías-Barragán J, Sandoval-Rodríguez A, Navarro-Partida J, etal.Themultifacetedroleofpirfenidoneanditsnovel tar-gets.FibrogenesTissueRepair.2010;3:16,http://dx.doi.org/ 10.1186/1755-1536-3-16.
7.Méndez-SánchezN,VillaAR,Chavez-TapiaNC,etal.Trendsin liverdiseaseprevalenceinMexicofrom2005to2050through mortalitydata.AnnHepatol.2005;4:52---5.
8.Abdallah HM, Ezzat SM, El Dine RS, et al. Corrigendum to ‘‘Protective effect of Echinops galalensis against CCl4
-induced injury on the human hepatoma cell line (Huh7)’’
[Phytochem.Lett.6(2013)73-78].PhytochemLett.2013;6:471, http://dx.doi.org/10.1016/j.phytol.2013.06.001.
9.BhaargaviV,JyotsnaGSL,TripuranaR.Areviewon hepatopr-tectiveactivity.IntJPharmSciRes.2007;5:690---702.
10.FernandoCD,SoysaP.Totalphenolic,flavonoidcontents, in-vitro antioxidant activities and hepatoprotective effect of aqueousleafextractofAtalantiaceylanica.BMCComplement AlternMed.2014:1---8.
11.Qureshi NN, Kuchekar BS, Logade NA, et al. Antioxidant and hepatoprotective activity of Cordia macleodii leaves. SaudiPharm J: OffPubl Saudi Pharm Soc. 2009;17:299---302, http://dx.doi.org/10.1016/j.jsps.2009.10.007.
12.HiraganahalliBD,ChinampudurVC,DetheS,etal. Hepatopro-tectiveandantioxidantactivityofstandardizedherbalextracts. PharmacognMag.2012;8:116---23.
13.Tanikawa K, Torimura T. Studies on oxidative stress in liver diseases: important future trends in liver research. Med Mol Morphol. 2006;39:22---7, http://dx.doi.org/10.1007/ s00795-006-0313-z.
14.De la MorenaGS, Martínez Pérez J. Efecto hepatoprotector inducidoporel flavonoide Astilbinafrente a un modelo ani-maltratadocontetraclorurodecarbono.RevCubaPlantMed. 1999;1:36---9.
15.Ai G, Liu Q, Hua W, et al. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot(L.)Medic:invitroand invivostudies.J Ethnophar-macol. 2013;146:794---802, http://dx.doi.org/10.1016/j.jep. 2013.02.005.
16.Ahmad F, Tabassum N. Experimental models used for the study of antihepatotoxic agents. J Acute Dis. 2012;1:85---9, http://dx.doi.org/10.1016/S2221-6189(13)60021-9.
17.KumarE,SusmithaK,SwathyB,etal.Areviewonliverdisorders andscreeningmodelsofhepatoprotectiveagents.IntJAllied MedSciClinRes.2014;2:136---50.
18.KashawV,NemaA,AgarwalA.Hepatoprotectiveprospectiveof herbaldrugsandtheirvesicularcarriers---areview.IntJRes PharmBiomedSci.2011;2:360---74.
19.PatilBR,BamaneSH,KhadsareUR.Invitroprotectionof hepa-tocytes by Alocasia macrorrhiza leaf juiceagainst CCl4 and tylenol mediated hepatic injury. Int J Pharm Appl. 2011;2: 122---7.
20.Vodovotz Y, Kim P, Bagci E, et al. Inflammatory modula-tion of hepatocyte apoptosis by nitric oxide: in vivo, in vitro, and in silico studies. Curr Mol Med. 2004;4:753---62, http://dx.doi.org/10.2174/1566524043359944.
21.Olinga P, Schuppan D. Precision-cut liver slices: a tool to model the liver ex vivo. J Hepatol. 2013;58:1252---3, http://dx.doi.org/10.1016/j.jhep.2013.01.009.
22.Gandolfi JA, Wijeweera J, Brendel K. Use of precision-cut liver slices as an in vitro tool for evaluating liver func-tion. Toxicol Pathol. 1996;24(1):58---61, http://dx.doi.org/ 10.1177/019262339602400108.
23.Guillouzo A. Liver cellmodels inin vitrotoxicology.Environ HealthPerspect.1998;106.
24.Van de Bovenkamp M, Groothuis GMM, Meijer DKF, et al. Liver fibrosis in vitro: cell culture models and precision-cut liver slices. Toxicol In Vitro. 2007;21:545---57, http://dx.doi.org/10.1016/j.tiv.2006.12.009.
25.EbadollahiNatanziA,GhahremaniMH,MinaeiB,etal.An exper-imental model for study ofthe hepatoprotectiveactivity of Nasturtiumofficinale(Watercress)againstacetaminofen toxic-ityusinginsituratliversystem.EurJSciRes.2009;38:556---64. 26.Roth RA, Ganey PE. Intrinsic versus idiosyncratic drug-induced hepatotoxicity --- two villains or one? J Pharma-colExp Ther.2010;332(3):692---7, http://dx.doi.org/10.1124/ jpet.109.162651.wisdom.
MedChem.2009;16(23):3041---53,http://dx.doi.org/10.2174/ 092986709788803097.
28.RajeshA,VijayK,PraveshKS,etal.Hepatoprotectivemodels andscreeningmethods:areview.JDiscovTher.2014;2:49---56. 29.RobinS,SunilK,RanaAC,etal.Differentmodelsof hepato-toxicityabsrelatedliverdiseases:areview.IntResJPharm. 2012;3:86---95.
30.Vasanth PR, RaghuHC, VijayanP, etal. In vitroand in vivo hepatoprotective effects of the total alkaloid fraction of Hygrophila auriculata leaves. Indian J Pharmacol. 2010;42: 99---104.
31.Zhou G, Chen Y, Liu S, et al. In vitro and in vivo hepato-protective and antioxidant activity of ethanolic extract from Meconopsis integrifolia (Maxim.) Franch. J Ethnophar-macol. 2013;148:664---70, http://dx.doi.org/10.1016/j.jep. 2013.05.027.
32.Shailajan S, Joshi M, Tiwari B. Hepatoprotective activity of
Parmeliaperlata(Huds.)Ach.againstCCl4inducedliver
tox-icity in Albino Wistar rats. J Appl Pharm Sci. 2014;4:70---4, http://dx.doi.org/10.7324/JAPS.2014.40212.
33.Wang A-Y. Gentiana manshurica Kitagawa prevents acetaminophen-induced acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway. World J Gastroenterol. 2010;16:384,http://dx.doi.org/10.3748/wjg.v16.i3.384. 34.McGill MR, Sharpe MR, Williams CD, et al. The
mecha-nism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclearDNAfragmentation.JClinInvest.2012;122:1574---83, http://dx.doi.org/10.1172/JCI59755.
35.Jaeschke H, Gores GJ, Cederbaum AI,et al. Mechanisms of hepatotoxicity.ToxicolSci.2002;176:166---76.
36.SimeonovaR, Kondeva-BurdinaM,VitchevaV,etal. Somein vitro/invivochemically-inducedexperimentalmodelsofliver oxidative stress in rats. Biomed Res Int. 2014;2014:706302, http://dx.doi.org/10.1155/2014/706302.
37.LimHK,KimHS,ChoiHS,etal.Effectsofacetylbergeninagainst
d-galactosamine-inducedhepatotoxicityinrats.PharmacolRes.
2000;42:471---4,http://dx.doi.org/10.1006/phrs.2000.0730. 38.Raj PV, Nitesh K, Prateek J, et al. Effect of lecithin
on d-galactosamine induced hepatotoxicity through
mito-chondrial pathway involving Bcl-2 and Bax. Indian J Clin Biochem.2011;26:378---84, http://dx.doi.org/10.1007/s12291-011-0155-x.
39.Wang C,Wang J, Lin W, et al. Protective effectof hibiscus anthocyaninsagainsttert-butylhydroperoxide-inducedhepatic toxicityinrats.FoodChemToxicol.2000;38:411---6.
40.Hwang J-M, Wang C-J, Chou F-P, et al. Protective effect of baicalin on tert-butyl hydroperoxide-induced rat hepa-totoxicity. Arch Toxicol. 2005;79:102---9, http://dx.doi.org/ 10.1007/s00204-004-0588-6.
41.AkhtarT,SheikhN.Anoverviewofthioacetamide-induced hep-atotoxicity.ToxinRev.2013;32:43---6.
42.LowTY,LeowCK,Salto-TellezM,etal.Aproteomicanalysisof thioacetamide-inducedhepatotoxicityandcirrhosisinrat liv-ers.Proteomics.2004;4:3960---74, http://dx.doi.org/10.1002/ pmic.200400852.
43.Moustafa AHA, Ali EMM, Moselhey SS, et al. Effect of coriander on thioacetamide-induced hepatotoxicity in rats. ToxicolIndHealth.2012;30:621---9,http://dx.doi.org/10.1177/ 0748233712462470.
44.HenryJB,Ernard.Laboratorio,vol.20.Marbán;2007. 45.DonfackJH,FotsoGW,Ngameni B,etal.Invitro
hepatopro-tective and antioxidant activities of the crude extract and isolated compounds from Irvingia gabonensis. Asian JTradit Med.2010;5:79---88.