Honey sweet honey: does it have a place in canine dermatology?
Texto completo
(2) HONEY SWEET HONEY: DOES IT HAVE A PLACE IN CANINE DERMATOLOGY?. Ana Margarida Pedroso de Oliveira. Doctoral Thesis. Departament de Medicina I Cirurgia Animals Facultat de Veterinària Universitat Autònoma de Barcelona 2016. 1.
(3) HONEY SWEET HONEY: DOES IT HAVE A PLACE IN CANINE DERMATOLOGY?. Ana Margarida Pedroso de Oliveira. Doctoral Thesis DIRECTORS: Mar Bardagí Admetlla y Lourdes Migura Garcia. Departament de Medicina i Cirurgia Animals Facultat de Veterinària Universitat Autònoma de Barcelona. Maig 2016. 2.
(4) 3.
(5) ACKNOWLEDGEMENTS First of all, I am very grateful to my family for their constant encouragement and support along the way. My deepest thanks extends to my personal and family friends that help me in completing this work.. I express my sincere thanks to my directors for their exceptional guidance and support, Dra Mar Bardagí Admetlla, docente del Departamento de Medicina y Cirugía Animales de la Universidad Autònoma de Barcelona and Dr Lourdes Migura Garcia, Researcher at IRTA.. My gratitude to Professora Laurentina Pedroso, Director Faculdade de Medicina Veterinária, Universidade Lusófona de Humanidades e Tecnologias, Doutor Pedro Faísca, Director of the Clinical Laboratory, Doutora Margarida Alves, Doutor Daniel Murta and Dra Ana Lúcia Rodrigues for providing me all necessary working support and facilities. I extend my sincere thanks to all faculty members and laboratory staff that continuos supported the study. I extremely grateful to my exceptional microbiology student, Dra Joana Devesa, who helped me along this venture.. My heartfelt gratitude to Dr Joost Postmes, Director of Triticum Company, Dr António Serra Nunes and Dra Isabel Pimenta from Biolotus Biotechenology Lda for believing and helping to support my project.. My sense of gratitude to one and all, who indirectly, gave me a helping hand at Àlamos Residency: Dra Teresa Ferro, Dra Manuel Soromenho Marques, Eng. Helena Forte, Eng. Margarida Marcelino, Dra Amparo Guirones, Dra Ana Filipa Rosário, Dra Madalena Brito, Inês Brandão, all residents and staff. My gratitude to the private clinic IMV Olaias: Dr Nuno Gaspar, Dr Luis Morais, Dra Susana Faria and Anabela Barroqueiro, owners and their dogs. Muito obrigada! Gracias! Thank you! 4.
(6) 5.
(7) INDEX. 1. ABREVIATIONS. 8. 2. SUMMARY. 10. 3. INTRODUCTION. 13. 3.1 Honey sweet honey. 14. 3.1.1 How bees produce honey?. 14. 3.1.2 Honey composition. 15. 3.1.3 Medical honey properties. 16. 3.1.4 Antibacterial and antifungal properties of the honey. 17. 3.1.5 Should we be concern about honey resistance?. 19. 3.1.6 Medical uses of honey in medicine. 19. 3.2 Staphylocococcus pseudintermedius. 23. 3.2.1 Characteristics of S. pseudintermedius. 23. 3.2.2 Canine diseases caused by S. pseudintermedius. 25. 3.2.3 Antibiotic resistance of S. pseudintermedius. 28. 3.2.4 Zoonotic aspects of S. pseudintermedius. 30. 6.
(8) 3.3 Malassezia pachydermatis. 32. 3.3.1 Characteristics of M. pachydermatis. 32. 3.3.2 Pathogenesis of M. pachydermatis. 32. 3.3.3 Clinical presentation M. pachydermatis. 34. 3.4 Susceptibility of M. pachydermatis to antimicotics. 35. 4. HYPOTHESIS. 38. 5.OBJECTIVES. 39. 5.1 General objectives. 40. 5.2 Specific objectives. 41. 6. STUDY 1. 43. 7. STUDY 2. 57. 8. STUDY 3. 72. 9. GENERAL DISCUSSION. 82. 10. CONCLUSION. 102. 11. REFERENCES. 104. 12. ANNEX. 144. 7.
(9) 1. ABREVIATIONS. 8.
(10) CFU – colonies forming units. CLS – chlorhexidine. CLSI - Clinical and Laboratory Standards Institute. FC – fusidic acid. FMV-UAB – Facultat de Medicina Veterinaria, Universitat Autonoma de Barcelona. FMV – ULHT – Facultat de Medicina Veterinaria, Universidade Lusofóna de Humanidades e Tecnologias. HBO – L-Mesitran Soft®, Triticum, The Nederlands. HO – honey that composes L-Mesitran Soft®. ISCAD - International Society for Companion Animal Infectious Diseases. LH – laboratory honey. MBC – minimal bactericidal concentration. MDR – multidrug-resistant Staphylococcus pseudintermedius. MFC – minimal fungicidal concentration. MGO – methylglyoxal. MH – manuka honey. MIC – minimal inhibitory concentration. MSSA – methicillin-susceptible Staphylococcus aureus. MSSP – methicillin-susceptible Staphylococcus pseudintermedius. MRSA – methicillin-resistant Staphylococcus aureus. MRSP – methicillin-resistant Staphylococcus pseudintermedius. TCS – triclosan. UTI - urinary tract infections.. 9.
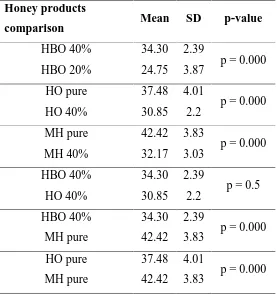
(11) 2. SUMMARY. In our days, honey is considered an effective, economic and widely available treatment for infected wounds. Manuka honey (MH) and honey-based products are marketed for medical practitioner to prescribe safe, effective and standardised products. More recently, honey has been rewarded as a possible potent topical antibiotic. In the present doctoral thesis, we characterized the multiresistance pattern of thirty methicillin-susceptible Staphylococcus pseudintermedius (MSSP) and thirty methicillinresistant Staphylococcus pseudintermedius (MRSP) isolated from dogs with canine pyoderma in referral hospitals in Spain and Portugal. Our results indicate a high percentage of multiresistant isolates (67%) in the MRSP group. We showed that multiresistance can be problematic due to limited antibiotic range and the use of antibiotics with severe side-effects like rifampicin, amikacin and chloramphenicol. In this case or, to prevent development of multiresistant bacteria, topical antimicrobial options may be recommended. MH and a honey-based gel with 40% medical honey (HBO) were investigated for their in vitro antimicrobial activity against MRSP, MSSP and Malassezia pachydermatis. Minimal bactericidal concentration (MBC) was established at 20% for MSSP and MRSP for HBO and MH. Both products revealed a hydrogen peroxide independent activity. Minimal fungicidal concentration (MFC) was defined at 10% for HBO and 40% for MH. We also documented susceptibility of the bacteria isolates against fusidic acid antibiotic, a fusidic acid cream, chlorhexidine and triclorsan. In case of M. pachydermatis the isolates were also tested against voriconazol and a clotrimazol cream. Both MH and HBO demonstrated a good bactericidal and fungicidal activity. Fusidic acid antibiotic, fusidic acid cream, chlorhexidine and trichlorsan also demonstrated a good antimicrobial activity against St. pseudintermedius, as well as voriconazol and clotrimazol cream against the yeasts. Based on this thesis, we conclude that manuka honey and a gel with 40% medical honey are effective against against M. pachydermatis and S. pseudintermedius independently of their meticillin-resistance.. 10.
(12) RESUMEN. Actualmente se considera que la miel es una alternativa efectiva, económica y ampliamente disponible para el tratamiento de heridas infectadas. La miel Manuka (MM) y otros productos con miel están disponibles para los facultativos médicos para prescribir tratamientos seguros, efectivos y estandarizados. Más recientemente, la miel se está considerando como a un posible potente antibiótico tópico. En esta tesis doctoral se ha caracterizado el patrón multiresistente de 30 cepas de Staphylococcus pseudintermedius susceptibles a meticilina (MSSP en sus siglas in inglés) y 30 cepas de Staphylococcus pseudintermedius resistentes a meticilina (MRSP en sus siglas in inglés). Todas las muestras fueron aisladas de perros con infección bacteriana cutánea visitados en hospitales de referencia de España y Portugal. Los resultados indicaron un elevado porcentaje de bacterias multiresistentes (67%) en el grupo de los MRSP. Este patrón de resistencia implica muy pocas opciones terapéuticas y el uso de antibióticos con efectos secundarios severos, como la rifampicina, la amicacina o el cloranfenicol. Es en estas situaciones, o para prevenir la aparición de bacterias multiresistentes, que el uso de un producto antimicrobiano tópico puede indicado. En la presente tesis doctoral hemos investigado la MM y un gel con un 40% de miel (HBO en sus siglas en inglés) para evaluar su efecto antibacteriano y antimicótico in vitro frente MRSP, MSSP y Malassezia pachydermatis. La concentración bactericida minima (MBC en sus siglas en inglés) del HBO y la MM ha sido del 20% para MSSP y MRSP. Ambos productos han demostrado también una actividad antimicrobiana independiente del peroxide de hidrógeno. La concentración minima fungicida (MFC en sus siglas en inglés) para el HBO se ha definido al 10% y al 40% para la MM. En el caso de las bacterias, también se ha investigado su susceptibilidad frente al antibiótico ácido fusídico, la clorhexidina y el triclorsan. Y en el caso de la Malassezia, también se ha testado el voriconazol y una crema de clotrimazol. El ácido fusídico, la clorhexidina y el triclorsan también han demostrado tener muy buena actividad antimicrobiana in vitro contra S. pseudintermedius, así como el clotrimazol y el voriconazol frente las levaduras. Podemos concluir de esta tesis doctoral que la MM y HBO son efectivos frente MRSP, MSSP y Malassezia pachydermatis. 11.
(13) RESUM Actualment es considera que la mel és una alternativa efectiva, econòmica i àmpliament disponible per al tractament de ferides infectades. La mel de Manuka (MM) i altres productes amb mel estan disponibles per als facultatius mèdics per prescriure tractaments segurs, efectius i estandarditzats. Més recentment, s’està considerant la mel com a un possible potent antibiòtic tòpic. En aquesta tesi doctoral s’ha caracteritzat el patró multiresistent de 30 soques d’Staphylococcus pseudintermedius susceptibles a meticil·lina (MSSP en anglès) i 30 soques d’Staphylococcus pseudintermedius resistents a meticil·lina (MRSP en anglès). Totes les mostres es van aïllar de gossos amb infecció bacteriana cutània visitats en hospitals de referència d’Espanya i Portugal. Els resultats han indicat un alt percentatge (67%) de bactèries multiresistents en el grup dels MRSP. Aquest patró de resistència impolica molt poques opcions terapèutiques i l’ús d’antibiòtics amb efectes secundaris severs, com la rifampicina, l’amicacina o el cloramfenicol. És en aquestes situacions, o per tal de prevenir l’aparció de bactèries multiresistents, que l’ús d’un producte antimicrobià tòpic pot estar indicat. En aquesta tesi doctoral hem investigat els efectes antimicrobians i antimicòtics de la MM i un gel amb un 40% de mel (HBO en anglès) contra MRSP, MSSP i Malassezia pachydermatis. La concentració bactericida mínima (MBC en anglès) del HBO i la MM ha estat del 20% per MSSP i MRSP. Ambdós productes han demostrat també una activitat antimicrobiana independent del peròxid d’hidrogen. La concentració mínima fungicida (MFC en anglès) per l’HBO s’ha definit al 10% i el 40 % per la MM. En el cas de les bactèries, també s’ha investigat la susceptibilitat davant l’antibiòtic àcid fusídic, la clorhexidina i el triclorsan. I en el cas de la Malassezia, també s’ha testat el voriconazol i una crema de clotrimazol. L’àcid fusídic, la clorhexidina i el triclorsan també han desmostrat tenir una bona activitat antimicrobiana in vitro contra S. Pseudointermedius. I el clotrimazol i el voriconazol davant els llevats. Podem concloure que la MM i el HBO són efectius davant MRSP, MSSP i Malassezia pachydermatis.. 12.
(14) 3. INTRODUCTION. 13.
(15) 3.1 Honey sweet honey 3.1.1 How bees produce honey? Honey has been a natural food source to human civilizations for centuries, being early Neolithic farmers the first documented consumers (Roffet-salque et al. 2015). According to the International Foods Standards OMS/FAO, honey is a natural sweet substance produced by honey bees from the nectar of plants which the bees collect, transform by combining with specific substances of their own, deposit, dehydrate, store and leave in the honey comb to ripen and mature (Codex Alimentarius Commission FAO/OMS 2001).. Bee colonies are one of the most organized groups in the animal kingdom. The workers collect nectar from the flowers and processor bees transform the nectar in honey, all of them females. Workers are older when compared to processors because they lack the necessary enzymes to process nectar. The drone bees are the males in the colony and their task is to fertilize the queen bee. The queen bee lays up to 2000 eggs a day creating the work force of the colony (Langstrom 1853).. Bees produce honey because it provides a good food source for the colony during winter. In order to produce honey, worker bees collect nectar from flower blossoms with their tongue and store it in the honey stomach. When the stomach is full, the bee returns to the hive and regurgitates the nectar directly into a processor bee mouth or into a honeycomb cell. A processor bee stores the nectar in the honey stomach. In the stomach, the nectar sucrose and complex sugars are converted into fructose and glucose by enzymes like invertase and amylase. Honey is then regurgitated into a honeycomb cell and sealed with wax. The wax allows the water to evaporate which, along with fan effect of the bee’s wings and the warm temperature inside the hive, gives honey its characteristic thick texture (Langstrom 1853).. 14.
(16) 3.1.2 Honey composition Honey is naturally rich in simple sugars and with a lower content of proteins, amino acids, vitamins, antioxidants, aromatic substances, minerals and organic acids (da Silva et al. 2016; Alqarni et al. 2016). The floral source, components of the honey and water content determine the physical characteristics of each type of honey, such as color, smell, taste, viscosity, solubility and conservation (Escuredo et al. 2013).. Honey is composed of roughly 68% of sugars and 17% of water with slight variations depending on the botanical source (Escuredo et al. 2013). In Europe, the honey marketed for human consume should meet the following criteria: no less than 60g/100g of fructose and glucose content in blossom honey; no more than 5g/100g of sucrose; no more than 20% moisture content; no more than 0.1g/100g of water-insoluble content; no less than 8 diastase activity (European Union 2002). Additional criteria are applied, for example, for conservation purposes. Minor constituents include enzymes, protein, aminoacids, organic acids, minerals, phenolic and volatiles compounds (da Silva et al. 2016).. Sweetness is due to monosaccharides like fructose, glucose, sucrose and maltose which are the main components of the honey (Escuredo et al. 2013). One of the characteristics that differentiate honey from other sweeteners is the presence of enzymes. These enzymes are produced by the bee during the conversion of nectar to honey in the nectar stomach. The most important enzymes are amylase, invertase and glucose oxidase. Honey possesses amylase, which hydrolyses starch into short-chain sugars like maltose. This is called the diastase activity of the honey (Boukraa et al. 2008). Invertase converts sucrose from nectar into glucose and fructose. Glucose oxidase is the enzyme that converts glucose to gluconolactone, which in turn yields gluconic acid and hydrogen peroxide. Catalase converts hydrogen peroxide into water and oxygen (Bogdanov et al. 2008).. 15.
(17) One of the main features about honey is the presence of natural antioxidants like phenolic compounds and flavonoids. These are biologically active substances with antioxidant and antinflammatory effect by scavenging free radicals in aerobic metabolisms (Alqarni et al. 2016; Erejuwa et al. 2012; Alvarez-Suarez et al. 2013; Pietta 2000). Antioxidants, along with nutritional components, vary between honeys depending on the floral source. Darker honeys are associated with a higher content of flavonoids (Escuredo et al. 2013; Bogdanov et al. 2008).. 3.1.3 Medical honey properties Honey is being increasingly used in the management of infected wounds where conventional pharmaceutical products are failing (French et al. 2005). Medical honey was licensed for wound care in Europe and America in 2004 and 2007, respectively (R. Cooper et al. 2010).. Most of the research for medical purposes has focused on the honey produced by the European honeybee Apis mellifera, although honey can also be produced by stingless bees. These species of bees (Apidae, Meliponini) are found in certain areas of the world like South America and Australia and include species such as Trigona carbonaria (Boorn et al. 2010; Souza et al. 2006).. Medical grade honey standard means that honey has been filtered, gamma-irradiated and handled under strict hygiene conditions to ensure a standardized product. Medical honey is sterilized by gamma-radiation to eliminate contaminating microorganisms including spores of Clostridium botulinum and Bacillus subtilis (Postmes et al. 1995). Gamma-radiation does not affect the antibacterial effect of the honey but heat sterilization does (Carnwath et al. 2014; Molan & Allen 1996).. Honey can be contaminated due to treatment of hives, for example, with antibiotics or by toxic substances. Chemical residues and pollution fallout observed in certain regions 16.
(18) of the world can be detected in honey (Rial-Otero et al. 2007; Postmes et al. 1993). Organic honey must be produced under the European directive for organic products, therefore avoiding toxic contamination (European Union 2007). It is recommend that honey used for medical purposes should be harvested without contamination (Feás & Estevinho 2011).. 3.1.4 Antibacterial and antifungal properties of the honey Several studies have demonstrated that honey has a remarkable activity against bacteria, fungi, virus and protozoa (Ansari et al. 2013; Fahim et al. 2014; Watanabe et al. 2014; Zeina et al. 1997).. Staphylococcus aureus is typically the most sensitive organism to honey and has been tested against several types of honey, including manuka honey, pasture honey and honey produced from stingless bees (Boorn et al. 2010; Cooper et al. 1999). Antibacterial activity has also been demonstrated against Staphylococcus coagulase negative, S. epidermitis and S. xylosus (French et al. 2005; Boorn et al. 2010). Other Gram-positive bacteria to which honey has antibacterial activity include Bacillus cereus, B. subtilis, Enterococcus faecalis and Listeria monocytogenes (Boorn et al. 2010).. Pseudomonas aeruginosa, which is another common pathogen in wounds and burns, has been also tested against medical honey (Al-Nahari et al. 2015; Roberts et al. 2012; Ballal et al. 2012). Other Gram-negative bacteria against which honey has antibacterial activity include: Stenotrophomonas species (Majtan et al. 2011), Escherichia coli (Blair et al. 2009; Boorn et al. 2010), Actinobacter baumanni (Boorn et al. 2010; George & Cutting 2007), Citrobacter freundi, Enterobacter cloacae, Klebsiella pneumoniae, Salmonella enterica ser. typhimurium, Serratia marcescens, Shigella sonnei, and Yersinia enterocolitica (Boorn et al. 2010).. 17.
(19) The antibacterial effect of honey is multifactorial due to several factors including osmotic effect due to high sugar content in a low volume of water, acidity due to the presence of gluconic acid (Karabagias et al. 2014), and hydrogen peroxide activity (Molan PC 1992). The antibacterial effect of manuka honey is associated with other components like methylglyoxal (Jenkins et al. 2011; Mavric et al. 2008) and leptosin also known as bee defensing, a glycoside which is a peptide found in the insects innate immune system (Kato et al. 2012).. The antibacterial effect can vary between different types of honey. Factors that affect honey antibacterial activity include floral source, different batches from the same floral source, geographical location, season in which honey was collected, treatment since harvesting, and age of the honey (Molan PC 1992). Monofloral honeys have better antibacterial effect compared to mixed botanical origin (Carnwath et al. 2014). A monofloral honey can be produced if the hive is in the center of a monofloral area, as bees in average fly 800 meters and no more than 6 kilometers (Hagler et al. 2011).. Honey has been reported to have antifungal activity against Aspergilus spp. and Penicillium spp. (Kacániová et al. 2011; Pradeep & Dhananjay 2012; AL-Waili et al. 2013). Yeast growth is also negativelly affected by honey and extensive studies have been performed with Candida albicans due to the impact in human health (Estevinho et al. 2011). Honey also has antifungal activity against other type of yeasts like Cryptococcus spp and Rhodotula spp (Ansari et al. 2013; Estevinho et al. 2011).. Medical honey is not considered a wound antiseptic. The criteria for antiseptic includes a quick effect against bacteria and fungi, acceleration of wound healing, local or systemic side-effects and cost effectiveness (Yaghoobi et al. 2013). The first criterion is not met because honey requires long time to achieve the antibacterial effect: the killing effect is only noticed at 1 hour, with a complete elimination after 3-24 hours for Grampositive bacteria, whereas for Gram-negative bacteria this time ranges from 4-6 hours up to 48 hours (N. Al-Waili 2004).. 18.
(20) 3.1.5 Should we be concerned about honey resistance? Recently, antibiotic resistant bacteria has been the leverage for research of natural alternatives like honey, aloe vera and tea tree oil (Falci, Sávia Perina Portilho Teixeira et al. 2015; Cataldi et al. 2015; Boorn et al. 2010). Currently, honey is recognized as an option against infections due to antibiotic resistant bacteria (Jenkins et al. 2011; AlNahari et al. 2015). French et al (2005) reported no difference between the antibacterial effect of manuka and pasture honey when resistant and susceptible isolates were compared. In another study, pasture and manuka honey revealed similar results, suggesting that there is no mechanism of resistance to either type of antibacterial activity of the honey (hydrogen peroxide or phytochemical), which is in contrast with the variation seen with the antibiotics (Cooper et al. 1999).. Several studies failed to induce honey resistance either in antibiotic-susceptible and antibiotic-resistant bacteria. Cooper et al (2010) demonstrated a lack of resistance after continuous exposure to manuka honey up to 28 days in two references (S. aureus and P. aeruginosa) and four clinical isolates (E. coli, methicillin resistant S. aureus (MRSA), P. aeruginosa and S. epidermitis). Another study, revealed that resistance to honey is not acquired when S. aureus and P. aeruginosa are exposed to continuous sublethal concentrations of honey (Blair et al. 2009). E. coli shows a unique transcriptional response when exposed to sublethal concentrations of honey, suggesting that mode of action of honey is different from antibiotics (Blair et al. 2009).. In sum, lack of resistance to honey may be due to the multifactorial antibacterial nature of the honey. Current findings do not rule out eventual future development of resistance, although it seems unlikely (Seckam & Copper 2013).. 3.1.6 Medical uses of honey in human medicine The first documented use of honey for medical purpose was found in a clay tablet from the Sumer civilization: "Grind to a powder river dust . . . and then knead it in water and 19.
(21) honey, and let oil and hot cedar oil be spread over it" (Sumerian clay tablet, c. 2000 B.C.). Honey was used in ancient Egypt to manage trauma wounds in battles and was mentioned in the first surgical papyrus: "Thou shouldst bind fresh meat upon [the wound] the first day, thou shouldst apply two strips of linen; and treat afterward with grease, honey, (and) lint every day until he recovers" (Smith surgical papyrus, 1700 BC).. Until the first part of the 20th century, honey dressings were commonly used for everyday wound care of patients with traumatic wounds, surgical incision sites, burns, sloughy wounds and pressure ulcers (Seckam & Copper 2013). With the introduction of antibiotics, honey became less used in clinical practice. Lately, the emergence of antibiotic resistant bacteria renewed the interested in honey as a safe and wide-spectrum antibacterial product for human use (Horn 2013). Honey conquered acceptance in wound healing treatment (Irish et al. 2011). An early review stated that honey is effective for the treatment of acute wounds and superficial partial thickness burns (Yaghoobi et al. 2013). A later Cochrane systematic review stated that honey appears to heal partial thickness burns faster than conventional treatment and infected postoperative wounds faster than antiseptics and gauze (Jull et al. 2015). Recommendations for other clinical applications could not be taken due to the low quality of the studies (Jull et al. 2015).. Honey has been reported to inhibit bacterial growth and may have a carioprotective effect in comparison to other sugars (Sela et al. 1998; Steinberg et al. 1996). Manuka honey was reported to prevent dental plaque development and gingivitis and both, manuka and acacia honey are used against halitosis (Pipicelli 2009; Shiga et al. 2010). Studies with electron microscope also have demonstrated that honey does not cause erosion of the tooth enamel (Sela et al. 1998). Oral lesions can be successfully treated with application of honey reducing significantly, the pain and duration of some inflammatory, vesiculobullous and ulcerative oral lesions (El-Haddad & Al-Shawaf 2013).. 20.
(22) Honey acts as an inhibitor of Helicobacter pylori, which is the causing agent of peptic ulcers and gastritis (al Somal et al. 1994; Manyi-Loh et al. 2010). An in vivo experiment performed with rats with induced gastric ulcers, suggested a healing effect associated with the ingestion of honey (Gharzouli et al. 2002; Mobarok Ali 2003). Several mechanisms of honey activity have been reported. Stimulation of the sensory nerves in the stomach that respond to capsaicin; antioxidant properties, which were observed in rats, preventing indomethacin-induced gastric lesions; and salivary reduction of nitrate to nitrite and intragastric formation of nitric acid (Beretta et al. 2010; Al-Swayeh & Ali 1998; Ali 1995). Dyspepsia due to H. pilori infection could be ameliorated if honey was included in the diet (Fahey et al. 2015). Antibacterial effect against this microorganism has been demonstrated with honey and propolis (al Somal et al. 1994; Nzeako & Al-Namaani 2006).. The administration of honey reduced the duration of bacterial diarrhoea in children with gastroenteritis (Haffejee & Moosa 1985). The oligosaccharides present in honey promote the growth of bifidobacteria and lactobacilli potentiating a prebiotic effect in rats and humans (Wahdan 1997; van den Berg et al. 2008).. Honey can also be used in the treatment of reflux oesophagus and heartburn (Math et al. 2013). Ingestion of considerable amounts of honey is associated with mild laxative effects due to the osmotic effect of fructose (Ladas et al. 1995).. Several studies have demonstrated that the administration of honey reduces cardiovascular risk factors, such as total cholesterol, low-density lipoproteincholesterol, triacylglycerol and C-reactive proteins, having no effect in increasing body weight, particularly in overweight patients (Yaghoobi et al. 2008; N. S. Al-Waili 2004a). Nitric oxide metabolites, which are cardiovascular risk indicators, can be present in honey. The nitrite concentration increased in different biological human fluids after honey ingestion (N. S. Al-Waili 2004a). Additionally, different reports have suggested the effect of honey in reducing cough and benefiting children sleep with upper respiratory tract infections (Warren et al. 2007; Oduwole et al. 2014). Moreover, there are also evidence of honey effectiveness in reducing persistent-post-infectious cough in adults (Ali Raeessi et al. 2013; Oduwole et al. 2014). Honey has an in vitro activity against the influenza virus, inhibiting its replication (Watanabe et al. 2014). 21.
(23) There are controversial reports on the effectiveness of honey concerning the prevention of hay fever, although there is a study that describes a positive effect in reducing or preventing the symptoms (Munstedt & Kalder 2010; Rajan et al. 2002). The ingestion of honey at high doses appears to improve the symptoms of allergic rhinitis and could be used as complementary treatment (Asha’Ari et al. 2013).. The beneficial effect of honey on radiation-induced mucositis, radiotherapy-induced skin reactions, hand and foot reactions to chemotherapy and surgical wounds has been reported (Bardy et al. 2008; Biswal et al. 2003; Moolenaar et al. 2006).. Finally, a recent study has described the nootropic effect of honey components, particularly polyphenols, on the brain (Mijanur Rahman et al. 2014). Honey as a carbohydrate source for athletes demonstrated an increase in the heart frequency and blood glucose level, thus enhancing performance (Kreider et al. 2002; Earnest et al. 2004). Other external applications for honey include action against eye pathologies, catheter infections, virus action on lips and genitals, boils and furuncles, muscle cramps, bruises and contusions and cosmetics (Johnson et al. 2005; N. S. Al-Waili 2004b; Burlando & Cornara 2013; Majtanova et al. 2015; Horn 2013). A positive effect of honey on ethanol intoxication in humans and rats has also been reported (Onyesom 2005; Ali 1991).. 22.
(24) 3.2 Staphylocococcus pseudintermedius 3.2.1 Characteristics of S. pseudintermedius The Staphylococcus genus (from the Greek σταφυλή, staphylē, "grape" e κόκκος, kókkos, "granule") is composed of Gram-positive bacteria, with a size between 0.8 to 1 µm. Under the microscope they appear similar to grape clusters (LSPN 2015). This genus includes 52 species and 28 subspecies, which are classified considering their genotypic differences, habitat and pathogeny (LSPN 2015). According to their ability to coagulate citrate in the plasma, by converting fibrinogen into fibrin, they are differentiated in coagulase-positive and coagulase-negative staphylococci.. S. intermedius was described for the first time in 1976 by V. Hajeck after being isolated from pigeons, dogs, minks and horses, allowing an important distinction between S. aureus and the new species (Devriese et al. 2009; Hajek 1976; Bannoehr & Guardabassi 2012; Bond & Loeffler 2012). Although Hajeck observed some heterogeneity between the strains described as S. intermedius, the species name remained unaltered until 2005, when Devriese proposed a new species named Staphylococcus pseudintermedius (Devriese et al. 2005). In 2007, a population genetic study using a multilocus sequence phylogenetic analysis, confirmed three distinct species, namely S. intermedius, S. pseudintermedius and S. delphini in isolates previously assumed to be S. intermedius (Bannoehr et al. 2007). These three species represent the S. intermedius group (SIG), and S. pseudintermedius was found to be the main pathogen involved in canine pyoderma (Bannoehr et al. 2007). Phenotypical characteristics do not allow to distinguish between S. pseudintermedius and S. delphini (Sasaki et al. 2007). Therefore, the routine identification of S. pseudintermedius in diagnostic laboratories is based on the fact that other species of the S. intermedius group are practically non-existent in dogs (Ross Fitzgerald 2009; Bannoehr & Guardabassi 2012). More recently, PCRRFLP was developed after sequence analysis of one of the loci, pta, which encodes the enzyme phosphoacetyltransferase and is a unique restriction site to S. pseudintermedius, which is an accurate and simple test that allows its identification (Bannoehr et al. 2009).. 23.
(25) S. pseudintermedius are mesophile, in other words, their optimal growth temperature is between 35-37ºC and have the ability to ferment carbohydrates. They are mainly facultative anaerobic, coagulase and catalase positive and have neither motility nor sporulation (Hajek 1976). They demonstrate rapid growth on most nutrient and enrichment mediums after 24 hour incubation. Colonies are round, raised, with a diameter of 1 to 2 mm and a colour that varies from nutrient agar white-grey to light yellow (Hajek 1976).. S. pseudintermedius is part of the normal cutaneous microflora, constituting about 90% of healthy dogs staphylococci, colonizing the skin, hair follicles and particularly mucosae like the nose, mouth and anus (Allaker et al. 1992; Griffeth et al. 2008; Fazakerley et al. 2009; Bannoehr & Guardabassi 2012). The skin of puppies is colonized by S. pseudintermedius shortly after birth, probably as a result of vertical transmission (Paul et al. 2014; Saijonmaa-Koulumies & Lloyd 2002). Clones can persist in the skin for long periods of time (Paul et al. 2014).. A study performed with 50 healthy dogs and 59 dogs suffering inflammatory skin disease detected that 68% (34/50) and 92% (48/59) of the healthy and affected dogs respectively, were colonized with S. pseudintermedius. Other identified species included S. aureus, S. schleiferi ssp. coagulans and S. schleiferi ssp. schleiferi (Griffeth et al. 2008). Another study reported that more than 94% of the S. pseudintermedius isolates recovered from cutaneous lesions of dogs with superficial pyoderma were genetically similar to the ones recovered from carriage sites (Pinchbeck et al. 2006).. S. pseudintermedius pathogenesis is not yet fully understood. Virulence factors include: enzymes such as coagulase, thermonuclease and proteases; surface proteins such as clumping factor and protein A; and toxins such as cytotoxins, exfoliative toxin and enterotoxins (Bannoehr & Guardabassi 2012). S. pseudintermedius cytotoxins, αhaemolysin and β-haemolysin, are known to cause haemolysis of rabbit erythrocytes and hot–cold haemolysis of sheep erythrocytes (Futagawa-Saito et al. 2006; Dziewanowska et al. 1996; Hajek 1976). A leukotoxin, Luk-1, encoded by the lukS and lukF genes, is also produced by S. pseudintermedius (Futagawa-Saito et al. 2004). The exfoliative toxin produced by S. pseudintermedius has a rounding effect on cultured epithelial cells. Dogs injected with it developed clinical signs similar to those observed 24.
(26) in pyoderma, such as erythema, exfoliation and crusting (Hesselbarth et al. 1994; Terauchi et al. 2003). Staphylococcal cell-wall-anchored proteins, such as microbial surface components recognizing adhesive matrix molecules have an important role in the adherence process (Ben Zakour et al. 2008). Some these anchoring proteins, which are named SpsA to SpsR, mediate adherence to proteins present in the extracellular matrix of the host, like fibrinogen, fibronectin, cytokeratin 10, elastin, collagen type I, vitronectin and laminin (Cree & Noble 1995; Geoghegan et al. 2009). Both, SpsD and SpsL present affinity to canine fibrinogen and in vitro assays reported that SpsD and SpsL are directly related to the adherence process to canine corneocytes (Bannoehr et al. 2011; Bannoehr et al. 2012). S. pseudintermedius adheres to the epidermal cells of healthy dogs and appears to show greater adherence in atopic dogs, being the later more heavily colonized (Simou et al., 2005; McEwan et al., 2006; Fazakerley et al., 2009).. S. pseudintermedius also produces biofilms (Futagawa-Saito et al. 2006; Singh et al. 2013), which are known to be resistant to antibiotics, environmental stress and macrophage phagocytosis (Olson et al. 2002; Shiau & Wu 1998). The production of biofilm, an extracellular polymeric matrix, is associated to the genes icaA and icaD, but further studies are necessary to evaluate this mechanism (Singh et al. 2013). No association has been found between the biofilm production and the presence of methicillin resistance genes, isolate source (infection site or colonization) or clonal complex (Singh et al. 2013). MRSP isolates are not more virulent when compared with MSSP isolates (Morris et al. 2006; Loeffler et al. 2007). However, infections with MRSP biofilm producers constitute an additional risk factor, since antibiotics cannot easily penetrate biofilm layers, therefore reducing the therapeutic options.. 3.2.2 Canine diseases caused by S. pseudintermedius S. pseudintermedius is the main cause of canine superficial and deep pyoderma, a frequent skin disease in dogs (Devriese et al. 2005). Moreover, S. pseudintermedius is also associated with otitis, surgical wound infections, urinary tract infections (UTI) and other acquired infections in dogs (Penna, Varges, Medeiros, et al. 2010; Rubin & Gaunt 2011; Windahl et al. 2015).. 25.
(27) Canine pyoderma is a common cause of canine skin disease, being superficial bacterial folliculitis the most common presentation (Hillier et al. 2014). Pyoderma can be classified in accordance to the depth of the lesions into: superficial, when the infection involves epidermis and/or hair follicles; and deep pyoderma when involves deeper tissues and possible furunculosis (Bloom 2014; Beco et al. 2013). Surface pyoderma involves the trauma of the surface of the epidermis only with colonization of the area by S. pseudintermedius (Bloom 2014; Beco et al. 2013).. Although S. pseudintermedius is the most common pathogen in canine pyoderma, other pathogens like S. aureus, S. schleiferi and S. hyicus are occasionally encountered (Frank et al. 2003; Cain CL1, Morris DO, O’Shea K 2011; Medleau et al. 1986). Rarely, coagulase-negative Staphylococci (S. epidermidis, S. xylosus, S. simulans and S hominis), Streptococcus canis and Pseudomonas aeruginosa can be found solely or in association with S. pseudintermedius (Fortin & Higgins 2001; Hillier et al. 2006; Medleau et al. 1986).. The most frequently isolated bacteria from dogs with otitis externa is S. pseudintermedius, constituting an important cause for antibiotic prescription (Lyskova et al. 2007; Lyskova et al. 2007). A study performed in 515 dogs affected with otitis externa reported S. pseudintermedius as the most commonly isolated bacteria, with 202 isolates recovered from pure culture or associated with other microorganisms (Lyskova et al. 2007). Another study also reported S. pseudintermedius as the most frequently isolated bacteria from 97 dogs with otitis externa (58.8%). One hundred and fifty one samples were obtained from dogs with unmedicated otitis, from which 35 isolates (85.7% of all the coagulase-positive staphylococci) were S. pseudintermedius, and the majority displayed multi-drug resistance (Penna, Varges, Medeiros, et al. 2010).. S. pseudintermedius is an important agent of canine UTI (Penna, Varges, Martins, et al. 2010; Hoekstra & Paulton 2002; Cohn et al. 2003). In a study involving 348 dogs, 70 (20.1%) of the isolates obtained from urine samples through catheterization, were staphylococci, being S. pseudintermedius the most common (23/70, 32.9%) (Penna, Varges, Martins, et al. 2010). S. pseudintermedius was described as the second most 26.
(28) isolated bacteria (60/623, 9.6%) in 623 urine cultures (Windahl et al. 2014). In another study with 1,478 urinary bacterial isolates, 147 were S. pseudintermedius, the third most common pathogen identified (Cohn et al. 2003). A case of UTI caused by MRSP was also described in a dog (Rubin & Gaunt 2011).. Nosocomial surgical wound infections in dogs and cats are also common and represent a risk factor for nosocomial transmission of MRSP in hospital environments. This emphasizes the need to implement good hygiene practices (Weese & van Duijkeren, 2010). In Sweden, a study analysed the pathogens involved in canine surgical wound infections and their susceptibility patterns. Out of 194 samples, the most prevalent was S. pseudintermedius (46%) and three isolates were MRSP (1%). No relation was found between the pathogen and classification of the surgical procedure, duration of hospitalization or depth of the surgical site infection (Windahl et al. 2015). Six MRSP were isolated from infected surgical wounds of five dogs and one cat at the Veterinary Microbiological Diagnostic Center of Utrecht University (van Duijkeren et al. 2011). The animals had undergone surgery at the same private veterinary practice. The isolates had a similar resistance profile as four samples recovered from the environment, four staff members and one healthy dog of one of the staff members, regularly present at the clinic. It was confirmed by Pulsed-field Gel Electrophoresis (PFGE), that all isolates were epidemiologically related. As the animals had no contact with each other, it was possible that the veterinary surgeon or the nurses were the source of infection, even though S. pseudintermedius rarely colonizes the human skin (van Duijkeren et al. 2008). Infection caused by MRSP was described after a joint prosthesis implantation in a dog, although, prior to the surgery, the animal was bitten by another dog (Miȩdzobrodzki et al. 2010).. Furthermore, S. pseudintermedius was identified as a complicating factor of immunomodulatory-responsive lymphocytic–plasmacytic pododermatitis in a study with 20 dogs (Breathnach et al. 2005). A fatal case of necrotizing fasciitis, with unknown source of infection and caused by MSSP was also described in a dog (Weese et al. 2009). S. pseudintermedius has also been isolated from abscesses in dogs (Hoekstra & Paulton 2002). A rare case of splenic abscess presenting septic peritonitis with concomitant. 27.
(29) infection by S. epidermidis and S. pseudintermedius in a German shepherd dog was also reported (Abdellatif et al. 2014).. 3.2.3 Antibiotic resistance of S. pseudintermedius In the past, most infections caused by S. pseudintermedius in dogs were effectively treated with empiric antibiotherapy with beta-lactams, macrolides or potentiated sulfonamide antibiotics (White 1996; Ganiere 2005). At least in Europe, multiresistance was extremely rare (Rantala et al. 2004; Guardabassi et al. 2004; Lloyd et al. 1996). Between 1987 and 1995, resistance to cefalexin, amoxicillin-clavulanic acid, oxacillin/methicillin and enrofloxacin had never been reported in the UK (Lloyd et al. 1996).. The first multiresistant MRSP isolates were reported at a dermatology referral centre in Germany in 2005 (Loeffler et al. 2007). Two strains of MRSP developed simultaneously in Europe and US with different resistant patterns. MRSP isolates were first reported in 1999 in North America and throughout Europe between 2005 and 2006, and is actually recognized as having a worldwide distribution (Onuma et al. 2012; Gortel et al. 1999; Schwarz et al. 2008; Perreten et al. 2010; Loeffler et al. 2007). The North America strain (ST68-C-t06-V) is still susceptible to chloramphenicol, rifampicin and amikacin, while the predominant MRSP clone in Europe, sequence type (ST71-Jt02-II–III) is, normally, resistant to beta-lactams, aminoglycosides, macrolides, lincosamides, tetracyclines, chloramphenicol, trimethoprim and fluoroquinolones, but remains susceptible to amikacin, fusidic acid, minocycline, rifampicin, vancomycin, teicoplanin and linezolid (Frank & Loeffler 2012; Perreten et al. 2010). This demonstrates the importance of recognizing MRSP susceptibility patterns, according to the clone distribution, in order to apply an effective antibiotherapy.. Methicillin, a semi-synthetic penicillinase-resistant penicillin of beta-lactams class, was introduced in 1959 to treat infections caused by betalactamase-producing staphylococci resistant to penicillin (Williams 1959). However, in 1961, methicillin resistance was documented in S. aureus (Barber 1961), from isolates recovered mainly in hospital environments (Hiramatsu et al. 2001). MRSA evolved to produce a defective penicillin28.
(30) binding protein mediated through the acquisition of the mecA gene. The mecA gene is part of a larger mobile genetic element known as the staphylococcal chromosomal cassette, which is integrated into the staphylococcal chromosome (Hiramatsu et al. 2001; Bond & Loeffler 2012). Therefore, methicillin resistance is directly linked to nonsusceptibility to virtually all beta-lactams (penicillins, potentiated amoxicillin and cephalosporins), and frequently also confers additional resistance to other antibiotics used in small animal practice (van Duijkeren et al. 2011; Bond & Loeffler 2012; Loeffler et al. 2007). As oxacillin has greater in vitro stability than methicillin, CLSI (Clinical and Laboratory Standards Institute) recommends the use of oxacillin disk to detect methicillin resistance and/or non-susceptibility to the beta-lactam antibiotic class for S. pseudintermedius (Bemis et al. 2009; CLSI 2013).. Both, the European Centre for Disease Prevention and Control and the Center for Disease Control and Prevention proposed that the isolates should be considered multiresistant if they display non-susceptibility to at least one antibiotic within three or more antibiotic groups (Magiorakos et al. 2012). In the MRSP case, and because oxacillin predicts resistance to beta-lactams, it can be considered multiresistant as it fits this description. However, if phenotypic methods are used for susceptibility testing, multiresistance is defined has resistance to three or more antibiotic classes (Schwarz et al. 2010; Coombs et al. 2004). The multiresistant phenotype is associated with genetic changes due to mobile genetic elements which in many cases encode for antibiotic resistance to several classes of antibiotics (Loeffler et al. 2013).. Generally, it is not advised to empirically switch antibiotic classes if treatment fails with first-line antimicrobials. In this case, culture and susceptibility testing should be performed before a second antibiotic is prescribed (Hillier et al. 2014). Differentiation between susceptible and resistant S. pseudintermedius strains based on the clinical picture is not possible, since MRSP is not more virulent than MSSP (Morris et al. 2006; Loeffler et al. 2007). Although resistance in staphylococci is not always associated with multidrug resistance, most of the MRSP isolates described in the literature display resistance to most clinically relevant antibiotics (Bond & Loeffler 2012). Reports from referral practices, with chronic or recurrent cases of pyoderma, in which previous antibiotherapy has been attempted, frequently report high levels of MRSP (Ben Zakour et al. 2012; Morris et al. 2006; Loeffler et al. 2007). 29.
(31) Nowadays, it is strongly recommended to general practitioners to follow the guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis developed by the Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases (ISCAID). These guidelines provide updated information for adequate treatment of canine folliculitis and rational use of antibiotics (Hillier et al. 2014).. 3.2.4 Zoonotic aspects of S. pseudintermedius The emergence of MRSP has renewed the interest in zoonotic implications of S. pseudintermedius. Humans are not the natural hosts for S. pseudintermedius and that explains the low impact of S. pseudintermedius in public health. However, it is unknown if S. pseudintermedius strains containing mobile genetic elements could represent a reservoir for the spread of resistant genes to the human commensal skin flora (van Duijkeren et al. 2011).. Recently, S. pseudintermedius has been implicated in occasional human infections (Hoovels, 2006 and Stegmann, 2010). Although S. pseudintermedius rarely colonizes the human skin, in those cases when individuals have regular contact with dogs, the colonization rate rises, particularly in the nasal cavities (Harvey et al. 1994; Goodacre et al. 1997; Stegmann et al. 2010; Guardabassi et al. 2004). It has been demonstrated that dog owners with dogs affected by deep pyoderma can carry the same genetic MRSP strain through nasal colonization as their pets, which supports an interspecies transmission (Guardabassi et al. 2004). Veterinarians in contact with infected animals also appear to have a higher risk of being MRSP nasal culture positive (Morris et al. 2010).. In 2006, it was identified the first case of S. pseudintermedius infection in a human patient with an implantable cardioverter-defibrillator (Van Hoovels et al. 2006). The first human infection caused by MRSP (ST71-t02-J with SCCmec II–III) was reported in 2010 from a patient with purulent sinusitis, but the definitive source of infection could not be determined (Stegmann et al. 2010). More recently, a S. pseudintermedius strain was described as a zoonosis and an opportunistic pathogen in a number of 30.
(32) patients with rhinosinusitis and immune dysfunction. In this report, some of the patients were dog owners of a dog affected with S. pseudintermedius infection (Kuan et al. 2016). S. pseudintermedius infection can also be associated with dog bite wounds (Talan et al. 1989; Tanner et al. 2000). More rarely, S. pseudintermedius have been associated with human infections like otitis externa (Tanner et al. 2000). Sasaki et al. (2007), also described prior to the reclassification, two strains in humans identified as S. intermedius (Sasaki et al. 2007). Recently, the ability of S. pseudintermedius to form antibiotic-resistant biofilms in human wounds has been reported (Pompilio et al. 2015).. Due to all the reasons stated above and ongoing research, it is possible that S. pseudintermedius infections are underdiagnosed in human medicine. It is now recommended that coagulase-positive Gram-positive cocci, not identified as S. aureus using routine microbiological methods, should undergo further identification to avoid mistaken identity (Viau et al. 2015). This is crucial as cefoxitin disks, used to determine S. aureus methicillin susceptibility (Bemis et al. 2009), do not allow an accurate identification of MRSP isolates which potentially can delay the treatment of human patients (Viau et al. 2015).. 31.
(33) 3.3 Malassezia pachydermatis 3.3.1 Characteristics of Malassezia pachydermatis Malassezia yeast is a unicellular eukaryotic symbiont that composes the microflora of the skin of several warm blooded species including humans, dogs and cats (Gaitanis et al. 2012). Currently, 14 species of Malassezia are recognized namely M. pachydermatis, M. furfur, M. globosa, M. obtusa, M. restricta, M. slooffiae, M. sympodialis, M. dermatis, M. nana, M. japonica, M. yamatoensis, M. equina, M. caprae being M. cuniculi the last species that has been identified (Cabañes et al. 2011; Gaitanis et al. 2012).. Malassezia is classified in the Phylum Basidiomycota, subphylum Ustilaginomycotina, class Exobasidiomycetes, order Malasseziales, and family Malasseziaceae (Baillon H. 1889). The genus Malassezia can be differentiated based on culture, biochemical and molecular testing (Kaneko et al. 2007; Mirhendi et al. 2005; Makimura et al. 2000). In humans, Malassezia causes pityriasis versicolor (Borgers et al. 1987; Hay & Midgley 2010) and can be implicated in the pathogenesis of atopic dermatitis (Hay & Midgley 2010). Malassezia, Cryptococcus and Rhodotula yeasts are among the few Basidiomycota that can become human pathogens (Saunders et al. 2012; Wirth & Goldani 2012).. Malassezia pachydermatis is a lipophilic yeast, commensal of the skin, mucosa and ear canal of the dog and cat, as well as other domestic and wild animals (Bond, SaijonmaaKoulumies, et al. 1995). M. pachydermatis has an optimal growth temperature of 37º C, although its growth is also possible between 25 and 40ºC (Guého et al. 1996; Morimoto et al. 2015). The isolates grow in either aerobic, microaerophilic or capneic conditions. Reduced growth is observed in anaerobic atmosphere (Morimoto et al. 2015).. M. pachydermatis is the only non-lipid-dependent Malassezia species while other species depend on lipids for their growth (Kaneko et al. 2007). This species was first described by Fred Weidman as Pityrosporum pachydermatis after isolation from an. 32.
(34) Indian rhinoceros (Rhinoceros unicornis) with a severe exfoliative dermatitis (Weidman 1925).. M. pachydermatis can be identified by sequence analysis of the large subunit (LSU) ribosomal RNA and by PCR-RFLP (Machado et al. 2011; Mirhendi et al. 2005). The internal transcribed spacer 1 (ITS-1) and the intergenic spacer 1 (IGS-1) of rDNA are useful for intraspecies diversity since multiple genotypic strains colonize dog skin (Sugita et al. 2005). There are three genotype groups for ITS-1 (A, B, C) and for IGS-1 (1,2,3). Strains are more diverse in ears than in the skin (Han et al. 2013). The same applies when diseased ears are compared to diseased canine skin (Han et al. 2013). Genotype C was found more commonly in canine healthy and diseased ears compared to healthy skin (Han et al. 2013). This difference can be associated with particular characteristics of the ear canal which includes a unique lipid and humid environment (Han et al. 2013).. 3.3.2 Pathogenesis of M. pachydermatis M. pachydermatis is the major pathogen of the skin and ear canals of the dog. In 1983, Dufait reported for the first time M. pachydermatis as the etiologic agent of generalized dermatitis in dogs (Dufait 1983). Particularly, M. pachydermatis is associated with atopic dermatitis, otitis externa and seborrheic dermatitis and contributes to worsening their clinical signs (Machado et al. 2011). Other species, including M. furfur, M. obtuse, M. globosa and M. sympodialis have been described in healthy and diseased skin and ears (Crespo et al. 2000; Raabe et al. 1998; Cafarchia et al. 2005).. Predisposition factors for the cutaneous proliferation of this commensal include allergic diseases, cornification disorders, bacterial skin infections, recent antibiotic therapy and long-term glucocorticoid administration (Plant et al. 1992). Genetic predisposition for Malassezia dermatitis appears to be important in certain breeds, including West Highland White Terriers, Basset Hounds, English Setters, Shih Tzus and American Cocker Spaniels (Bond et al. 1996; Mauldin et al. 1997). In the Basset Hound breed, yeast proliferation has been associated with a primary keratinization disorder (Power et al. 1992).. 33.
(35) M. pachydermatis is able to produce several virulence factors including esterases, lipases, lipoxygenases, proteases, hyaluronidases and chondroitinsulfatases. However, the phospholipase activity has been the most studied (Coutinho & Paula 2000; Cafarchia & Otranto 2004; Juntachai et al. 2009). Lipolytic enzymes are one of the virulence factors of Malassezia spp. In case of lipid-depend species, lipases hydrolyze myriastic acid, which serves as a precursor of long-chain fatty acids (Porro et al. 1976; Mancianti et al. 2001). M. pachydermatis has the highest secreted phospholipase activity among other species (Juntachai et al. 2009). It was postulated that hydrolization of glycerophospholipids of host cell membranes by phospholipase is involved in the pathogenesis of these species in canine skin lesions (Cafarchia & Otranto 2004) and otitis externa (Teramoto et al. 2015) .. There are several factors that favour Malassezia growth, such as humidity, high temperature and an environment rich in fat (Weiler et al. 2013). Additionally, the skin microflora, pH, salts, immune response and other physiological characteristics are also considered important in colonization by Malassezia (Cafarchia et al. 2008).. Adherence is the ability to attach to cells and tissues, allowing colonization and infection, permitting the microorganism to resist physical forces that would result in its removal from the host (Guillot & Bond 1999; Born & Havenith 2009). Studies propose that the adherence process is altered regarding lipid-dependant and non-lipid-dependant strains and the difference in their fatty acid composition (Florek et al. 2014; Schechtman et al. 1995). The adherence to keratinocytes is a key contributing factor in the expression of proinflammatory cytokines in humans, and it is suggested as the main contributing factor for Malassezia dermatitis in dogs (Akaza et al. 2012).. 3.3.3 Clinical presentation of Malassezia pachydermatis M. pachydermatis lives in healthy skin and mucosa of the dog. Although it is commonly isolated from asymptomatic skin of the external ear canal, anus, perioral and interdigital skin (Baxter 1976), sometimes it can act as an opportunistic pathogen (Bond & Lloyd 1997).. 34.
(36) Malassezia dermatitis lesions in dogs are normally intensely pruritic and the main primary lesion is erythema (Morris 1999). Secondary lesions normally consist in excoriations, hyperpigmented areas, erythematous lesions, varying degrees of traumatic alopecia and scaling that normally affect the ventral abdomen, ventral neck, face, axillae, feet, limbs, perineal regions and skin folds (Morris 1999; Bond 2010b). The lesions may be confined to one area or affect multiple regions (Morris 1999; Bond, 2010a; Bond 2010b). Dry skin or seborrhoea oleosa with waxy adherent scales is often observed in skin folds and interdigital areas and frequently accompanied by a yeasty odour (Morris 1999; Bond, 2010a). Chronic lesions are frequently accompanied by hyperpigmentation and lichenification of the area (Bond, 2010a). Lesions in the interdigital skin may progress to involve the claw folds, producing exudation and redbrown discoloration of the paronychia hairs or claws (Morris 1999; Bond, 2010a).. In dogs with otitis externa erythema of the external ear canal and pinnae is frequent and often accompanied by changes in the ear cerumen. It is observed an increase in the quantity and change to a brownish colouration commonly accompanied by pruritus. Stenosis, hyperpigmentation and calcification of the ear is associated with chronicity (Bond, 2010a; Bond 2010b).. 3.3.4 Susceptibility of M. pachydermatis to antimicotics Several types of antifungal drugs have been used to treat Malassezia dermatitis and otitis which includes azoles, allylamines and polyene macrolides (Gupta et al. 2000; Yurayart et al. 2013). Other antifungals include chlorhexidine (Young et al. 2012), piroctone olamine (Rème et al. 2005), salicylic acid (Ghibaudo & Graziano 2002), and selenium sulphide (Van Cutsem et al. 1990).. Azole drugs inhibit cytochrome enzyme P450 complex, including the 14α-demethylase enzyme. This enzyme converts lanosterol to ergosterol which is an essential component of the fungal cell membrane. The lack of ergosterol causes instability leading to cell death (Piérard et al. 2012). Several azoles have been reported to be active against M. pachydermatis which includes ketoconazole (Cafarchia, L. A. Figueredo, et al. 2012), itraconazole (Weiler et al. 2013), fluconazole (Cafarchia, L. a. Figueredo, et al. 2012), 35.
(37) miconazole (Peano et al. 2012), clotrimazole (Crowley & Gallagher 2014), climbazole (Schmidt 1997) and thiabendazole (Nascente et al. 2009).. Oral terbinafine has an in vivo antifungal activity against Malassezia pachydermatis, with no adverse effects being reported (Guillot et al. 2003; Rosales et al. 2005). Terbinafine is an allylamine and acts as a fungicidal and fungistatic against M. pachydermatis. The primary mechanism of action of terbinafine consists in the inhibition of the enzyme squalene epoxidase, which is responsible for the conversion of squalene in lanosterol. Ultimately, interferes in the pathway of ergosterol synthesis, impeding cell wall function. This also results in toxic intracellular squalene accumulation, ensuing in rapid death (Ryder 1985; Faergemann et al. 1996; McClellan et al. 1999; Leyden 1998).. Nystatin is a polyene macrolide produced by Streptomyces noursei that has a fungistatic and fungicidal activity. Nystatin binds to the ergosterol in the yeast cell membrane, changing the permeability and causing oxidative damage (Rezabek & Friedman 1992; Weiler et al. 2013; Bossche et al. 2003). Lyskova et al. (2007) and Yurayart et al. (2013) reported lack of resistant to nystatin, using the disk-diffusion susceptibility test in a group of dogs with healthy and seborrheic skin and healthy and otitic ears.. Antifungal systemic treatment can be associated with adverse effects reported in the dog, including hepatotoxicity (Mayer et al. 2008). Some of the drugs like ketoconazole may inhibit P450 cytochrome interfering with the metabolism of co-administered drugs (Rosales et al. 2005; Mayer et al. 2008). For this reason, topical therapy is commonly preferred as the unique treatment or associated with systemic drugs. In vivo activity of shampoos with miconazole formulations in dogs with seborrheic dermatitis associated with M. pachydermatis was assessed in several studies (Bond, Rose, et al. 1995; Bourdeau et al. 2011). Topical treatment with miconazole preparations in dogs with otitis externa was effective in reducing the number of yeast-cells (Bensignor & Grandemange 2006; Studdert & Hughes 1991; Rougier et al. 2005). Bond et al. (1995) led a randomized double-blinded parallel study comparing the efficacy of a 2% miconazole and 2% chlorhexidine shampoo with a 0,25% selenium sulfide shampoo in 33 Basset hounds. The patients where shampooed at a 3 day interval for 3 weeks, 16 of 36.
(38) them with the miconazole-chlorhexidine shampoo and 17 with selenium sulfide. The miconazole-chlorhexidine shampoo was more efficient in reducing clinical signs and M. pachydermatis counts, although the selenium sulfide shampoo was as effective in reducing the yeast counts. No adverse effects were reported (Bond, Rose, et al. 1995).. Climbazole is an imidazole that is incorporated in veterinary products in the form of shampoos or wipes (Cavana et al. 2015; Schmidt 1997). Schmidt (1997) evaluated the in vitro activity of climbazole against 40 M. pachydermatis isolates using microdilution broth methods and reported high levels of susceptibility. Bourdeau (2011), in a blinded randomized trial, compared the efficacy of a 2% chlorhexidine and 2% miconazole shampoo and a 3% chlorhexidine and 0,5% climbazole douxo-chlorhexidine in 16 dogs. The yeast count was significantly reduced with both shampoos, with no statistical difference between the groups.. Chlorhexidine is a bisbiguanide used as a biocide in antiseptic products as it has a broad-spectrum efficacy (Mueller et al. 2012; Mcdonnell & Russell 1999). The clinical efficacy of chlorhexidine in shampoo formulations in the treatment of dogs with M. pachydermatis dermatitis is reported in several studies (Bond, Rose, et al. 1995; Lloyd & Lamport 1999; Jasmin et al. 2003; Maynard et al. 2011). In vitro studies support these results (Young et al. 2012; Odore et al. 2000).. Piroctone olamine, also known has octopirox, is a member of the 1-hydroxy-2pyridones family that has an antifungal activity. It acts in the cell membrane by impairing the transport of essential substrates like leucine which causes instability and rupture (Frangi & Coli 2005). Bourdeau (2006), in a study with 20 Beagles colonized with M. pachydermatis, evaluated the effect of a diluted shampoo containing piroctone olamine applied on the skin and ear canals. Progressive antifungal activity was observed four days after product application, demonstrating that piroctone olamine is an effective alternative in the treatment of M. pachydermatis dermatitis. Rème et al. (2005) in an open clinical field trial, evaluated the clinical results of a shampoo containing ammonium lactate 10% and piroctone olamine 0.5% in a group of 16 dogs with M. pachydermatis dermatitis. Reduction of the clinical signs and yeast proliferation was significant.. 37.
(39) 4. HYPOTHESIS. Current scientific knowledge defines honey has a natural product with antibacterial and antifungal properties. The hypothesis of this work, was to determine the antimicrobial effect of manuka honey and a honey-based product against two canine skin pathogens: Staphylococcus pseudintermedius and Malassezia pachydermatis.. 38.
(40) 5. OBJECTIVES. 39.
(41) 5.1 General objectives. Honey has been used since ancient times in the treatment of infected wounds. The antibacterial effect has been proven against human bacterial and fungal pathogens that cause several types of skin conditions. The work in veterinary medicine is scarce and the emergence of methicillin-resistant S. pseudintermedius led to insights in topical, non-antibiotic based treatment options. The objectives of this doctoral thesis are to answer the following questions:. 1- Which is the antimicrobial susceptibility profile of S. pseudintermedius isolates obtained from dogs with pyoderma in a referral setting? Is multidrug resistance frequent?. 2- Manuka honey (MH) and a honey-based gel (HBO) are available for medical use. Are these products effective against MSSP and MRSP?. 3- Malassezia pachydermatis is a common fungal pathogen of the canine skin. Do MH and a HBO have a fungicidal activity against this yeast?. 40.
(42) 5.2 Specific objectives. Study 1: The aim of the study is to describe the phenotypic diversity of S. pseudintermedius, methicillin-susceptible (n=30) and resistant (n=30) isolated from dogs with pyoderma in a referral setting in Spain and Portugal. . Analyze clinical data of the patients (age, breed, sex, neuter status).. . Speciation of the isolates.. . Detection of the methicillin resistant mechanism (mecA gene) by PCR.. . Susceptibility testing to 14 antimicrobials.. . Identify the presence of multiresistance isolates (resistance to three or more antibiotic classes) among MRSP and MSSP groups.. Study 2: The aim of this study was to determine in vitro efficacy of HBO and MH against MSSP and MRSP. . Determine minimal bactericidal concentration (MBC) for both products.. . Assessment of the efficacy of the honey component of the gel (HO).. . Comparison of the bactericidal activity between HBO and MH.. . Determination of the hydrogen peroxide activity of the HBO, HO and. MH. . Assess the susceptibility of the isolates to fusidic acid.. . Determination of the MBC to fusidic acid cream (FC).. . Determination of MBC to biocides, chlorhexidine (CLX) and triclorsan. (TCS).. 41.
(43) Study 3: The aim of this study was to determine in vitro efficacy of a HBO and MH against M. pachydermatis. . Determine minimal antifungal concentration (MFC) of HBO and MH. against M. pachydermatis. . Assessment of the efficacy of the honey component of the gel. Characterize susceptibility of the isolates to voriconazole by disk. diffusion susceptibility test. . Determine MFC to a miconazole cutaneous solution.. 42.
(44) 6. STUDY 1. 43.
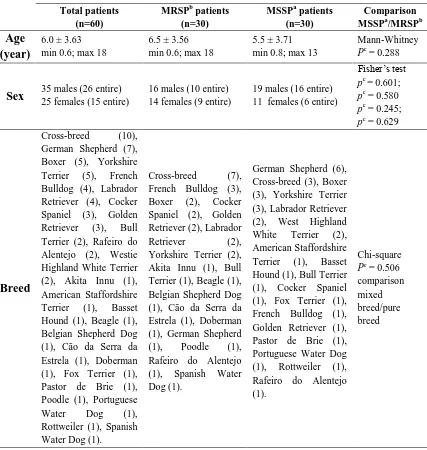
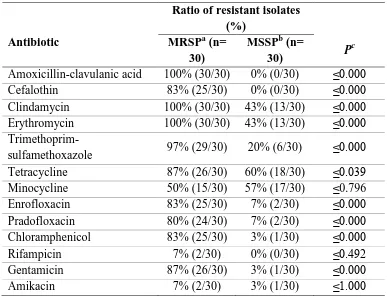
(45) Antibiotic susceptibility profile of Staphylococcus pseudintermedius isolated from canine pyoderma. Abstract Staphylococcus pseudintermedius is part of the normal microbiota of canine skin and mucosa. It is the most common bacterial pathogen associated with canine pyoderma, in addition to being a major cause for antibiotic prescription in small animal practice.. The aim of the present work is to evaluate the incidence of multiresistance (resistance to three or more antibiotic classes) in susceptible and methicillin-resistant S. pseudintermedius isolates, from dogs with superficial pyoderma in a referral setting from two hospitals in Portugal and Spain.. Sixty isolates of S. pseudintermedius were tested for oxacillin susceptibility by the Kirby-Bauer technique and divided into two groups: methicillin-resistant S. pseudintermedius (MRSP, 30/60, 50%) and methicillin-susceptible S. pseudintermedius (MSSP, 30/60, 50%). The isolates were tested for first and second tier antibiotics recommended by the Working Group of the International Society for Companion Animal Infectious Diseases guidelines (Hillier et al. 2014). PCR was used for speciation of the isolates and detection of the mecA gene.. Most of the MRSP isolates were multidrug-resistant (MDR, 67%) and were associated with resistance to methicillin (p = 0.000). All non-MDR isolates were MSSP. Among the MRSP, all isolates exhibited resistance to amoxicillin-clavulanic acid, clindamycin and erythromycin. High levels of resistance were observed to trimethoprimsulfamethoxazole (97%), tetracycline and gentamicin (87%), cefalothin and enrofloxacin (83%) followed by pradofloxacin (80%) and minocycline (50%). Low level of resistance was observed to chloramphenicol (17%), amikacin (7%) and rifampicin. (7%).. Only. one. MRSP. isolate. was. susceptible. trimethoprim-. sulfamethoxazole. Within the MSSP group, 60% and 57% of the isolates presented resistance to tetracycline and minocycline, respectively. Additionally, 43% of the 44.
(46) isolates were resistant to clindamycin and erythromycin, 20% to trimethoprimsulfamethoxazole, 7% to enrofloxacin and pradofloxacin and 3% to chloramphenicol, gentamicin and amikacin. None of them were resistant to amoxicillin-clavulanic acid, cefalothin and rifampicin.. The presence of methicillin-resistant isolates should alert the practitioner for MDR and, consequently, for limited antibiotic options.. Introduction In the nineties, most infections caused by S. pseudintermedius in dogs were effectively treated with empiric antibiotherapy with beta-lactams, macrolides or potentiated sulfonamide antibiotics (White 1996; Ganiere 2005). At least in Europe, multiresistance was extremely rare (Rantala et al. 2004; Guardabassi et al. 2004; Lloyd et al. 1996). Between 1987 and 1995, resistance to cephalexin, amoxicillin-clavulanic acid, oxacillin/methicillin and enrofloxacin had never been reported in the UK (Lloyd et al. 1996).. The first multiresistant MRSP isolates were reported at a dermatology referral centre in Germany in 2005 (Loeffler et al. 2007). Two strains of MRSP developed simultaneously in Europe and US with different resistant patterns. MRSP isolates were first reported in 1999 in North America and throughout Europe between 2005 and 2006, and is actually recognized as having a worldwide distribution (Onuma et al. 2012; Gortel et al. 1999; Schwarz et al. 2008; Perreten et al. 2010; Loeffler et al. 2007). The North America strain (ST68-C-t06-V) is still susceptible to chloramphenicol, rifampicin and amikacin, while the predominant MRSP clone in Europe, sequence type (ST71-Jt02-II–III) is, normally, resistant to beta-lactams, aminoglycosides, macrolides, lincosamides, tetracyclines, chloramphenicol, trimethoprim and fluoroquinolones; but remains susceptible to amikacin, fusidic acid, minocycline, rifampicin, vancomycin, teicoplanin and linezolid (Frank & Loeffler 2012; Perreten et al. 2010). This demonstrates the importance of recognizing MRSP susceptibility patterns, according to the clone distribution, in order to apply an effective antibiotherapy.. 45.
(47) The aim of the present work was to evaluate the multidrug-resistant profile of 30 MRSP and 30 MSSP isolates from two referral practices in Portugal and Spain.. Material and Methods Collection of the isolates Samples were obtained from 38 and 22 atopic dogs presented to the Dermatology Service of Faculty of Veterinary Medicine at Universidade Lusófona de Humanidades e Tecnologias and Universitat Autònoma de Barcelona, respectively, after diagnosis of bacterial folliculitis. Diagnosis was based on clinical signs and compatible cytological findings (neutrophils with intracellular cocci). Samples were collected either from intact pustules by puncturing with a 0.50x16 mm sterile needle (Braun, Germany) or by rubbing with an sterile swab the margin of epidermal collaretes. The material was collected using a sterile swab (Portagem Amies Agar, Copan S.P.A, Italy) followed by overnight incubation on blood agar and MacConkey agar at 37˚C. Isolates were characterized as Gram-positive cocci, catalase positive and coagulase positive and stored at -20˚C in a mixture of 15% glycerol and nutrient broth . A total of 60 S. pseudintermedius isolates (30 methicillin susceptible and 30 methicillin resistant) were included in the study.. Molecular characterization of the isolates: PCR for specification of the isolates: the isolates were identified as S. pseudintermedius, as described by Bannoehr (Bannoehr et al. 2009). For detection of mecA gene a multiplex PCR using the sequences mecA-1: 5'-GGGATCATAGCGTCATTATTC-3', mecA-2:. 5'-AACGATTGTGACACGATAGCC-3',. nuc-1:. 5'-. TCAGCAAATGCATCACAAACAG-3', nuc-2: 5'-CGTAAATGCACTTGCTTCAGG3',. 16S-1:. 5'-GTGCCAGCAGCCGCGGTAA-3'. and. 16S-2:. 5'-. AGACCCGGGAACGTATTCAC-3'. PCR was run according to the standard protocol (National Food Institute 2009).. 46.
(48) Methodology for antibiotic testing: Antibiotic susceptibility testing was performed in accordance with the Kirby-Bauer methodology described in CLSI-Vet 2014. The Mueller-Hinton agar plate was inoculated by streaking the swab three times over the entire agar surface, rotating the plate 60º each time to ensure an even distribution of the inoculum. Plates were incubated overnight at 37C. Antibiotics were chosen based on ISCAID guidelines for the treatment of bacterial folliculitis (Hillier et al, 2014). The inhibition halos were interpreted as susceptible or resistant using the diameters recommended by CLSI-Vet 2014 and, if not available, EUCAST (www.eucast.org). The following antibiotic disks were tested: oxacillin (1 µg), amoxicillin-clavulanic acid (20/10 µg), cefalothin (30 µg), clindamycin (2 µg), erythromycin (15 µg), trimethoprim-sulfamethoxazole (1.25/23.75 µg), tetracycline (30 µg), minocycline (30 µg), enrofloxacin (5 µg), pradofloxacin (5 µg; Mast Diagnostics, UK), chloramphenicol (30 µg), rifampicin (5 µg) , gentamicin (10 UI; Bio-Rad, France) and amikacin (30 µg). Staphylococcus aureus subsp. aureus (ATCC® 29213™, Oxoid, UK) strain was used as quality control as recommended by CLSI. All media and antibiotics were supplied by Oxoid ((Oxoid, Hampshire, UK) unless stated otherwise.. The inhibition halos were read with the naked eye and measured with a rule (Hudzicki, 2013). Multiresistance was defined as resistance to three or more classes of antibiotics (Schwarz et al. 2010; Coombs et al. 2004) and the isolates were classified into MDR or non-MDR. The following antibiotics were used to determine the MDR pattern: oxacillin, clindamycin, erythromycin, trimethoprim-sulfamethoxazole, tetracycline, enrofloxacin, chloramphenicol, rifampicin and gentamicin.. Statistical analysis was performed with Statistical Package for the Social Sciences 16.0 (IBM SPSS Chicago, IL).. 47.
Figure

Documento similar
Abstract: Transepidermal water-loss (TEWL), stratum-corneum hydration (SCH), erythema, elas- ticity, pH and melanin, are parameters of the epidermal barrier function and
Place all of the berries into a large bowl and toss gently to combine with the lemon juice and honey mixture.. Pour the coated berries into the pie
This work was based on the hypothesis that the expression profile of miRNAs in the testicular tissue of Klinefelter patients differs from that of the controls and at least some of
En el capítulo 8, se estudió el posible efecto de una miel artesanal junto con los distintos tipos de envasado (aire, vacío y atmósfera modificada) sobre la conservación de
The Dwellers in the Garden of Allah 109... The Dwellers in the Garden of Allah
3.3 STATCOM controllers design using HBMO In the proposed method, we must tune the STATCOM controller parameters optimally to improve overall system dynamic stability.. The two
The objective of the present work was to develop a soy protein-based edible coating with antioxidant activity and to evaluate the combined effect of selected edible
In the light of this gap in current knowledge, the objective of this study was to determine the effect of heat stress on milk production and quality by studying the effects of THI



