EDITORIAL
Acute pancreatitis at the beginning of the 21st century: The
state of the art
Alfredo F Tonsi, Matilde Bacchion, Stefano Crippa, Giuseppe Malleo, Claudio Bassi
doi:10.3748/wjg.15.2945 © 2009 The WJG Press and Baishideng. All rights reserved.
Alfredo F Tonsi, Department of General Surgery, East Surrey Hospital, Canada Avenue, Redhill, Surrey, RH1 5RH,
United kingdom
Matilde Bacchion, Stefano Crippa, Giuseppe Malleo, Claudio Bassi, Department of Surgery, University of Verona, 37134 - Verona, Italy
Author contributions: Tonsi AF contributed to manuscript preparation, manuscript editing and was the primary writer of the manuscript; Bacchion M, Crippa S and Malleo G reviewed the manuscript; Bassi C was involved in the conception of the editorial, manuscript editing and manuscript review.
Correspondence to: Claudio Bassi, MD, FRCS, Professor of Surgery, Department of Surgery, "G.B. Rossi" Borgo Roma Hospital, University of Verona, 37134 - Verona,
Italy. claudio.bassi@univr.it
Telephone: +39-45-8124553 Fax: +39-45-8201294 Received: April 17, 2009 Revised: May 9, 2009 Accepted: May 16, 2009
Published online: June 28, 2009
Abstract
Acute pancreatitis is an acute inflammatory disease of the pancreas which can lead to a systemic inflammatory response syndrome with significant morbidity and mortality in 20% of patients. Gallstones and alcohol consumption are the most frequent causes of pancreatitis in adults. The treatment of mild acute pancreatitis is conservative and supportive; however severe episodes characterized by necrosis of the pancreatic tissue may require surgical intervention. Advanced understanding of the pathology, and increased interest in assessment of disease severity are the cornerstones of future management strategies of this complex and heterogeneous disease in the 21st century.
© 2009 The WJG Press and Baishideng. All rights reserved.
Key words: Acute necrotizing pancreatitis; Systemic inflammatory response syndrome; Surgery; Pancrea tectomy; Minimal surgical procedures
Peer reviewers: Jens Werner, MD, Associate Professor, Department of General and Visceral Surgery, University of Heidelberg, INF 110, Heidelberg 69120, Germany; Rakesh Kumar Tandon, Professor, Pushpawati Singhania Research Institute for Liver, Renal and Digestive Diseases, Sheikh Sarai-Phase II, New Delhi 110017, India
Tonsi AF, Bacchion M, Crippa S, Malleo G, Bassi C. Acute
pancreatitis at the beginning of the 21st century: The state of the art. World J Gastroenterol2009; 15(24): 2945-2959 Available from: URL: http://www.wjgnet.com/1007-9327/15/2945.asp DOI: http://dx.doi.org/10.3748/wjg.15.2945
INTRODUCTION
The death of Alexander the Great (356-323 BC) at the age of 33 has been ascribed to acute necrotizing pancreatitis secondary to rich food and heavy alcohol consumption[1].
In 1856, the great French physiologist Claude Bernard (1813-1878) demonstrated the capacity of pancreatic secretions to digest proteins, carbohydrate and fat[2]. However, the first review on acute pancreatitis (AP) was
published by Reginald Huber Fitz (1843-1913) in 1889[3].
In his observation of 53 patients with clinical signs of
AP, he believed that the disease was a complication of
gastroduodenitis causing inflammation of the biliary duct.
Nevertheless, only in late 19th century did Chiari propose pancreatic autodigestion as the main pathological mechanism of the disease[4]. This theory facilitated the
understanding of the late 19th and early 20th century
clinicians in the pathophysiology of AP. In 1901, Eugen
Opie proposed the “common channel hypothesis”, which was based on the assumption that a gallstone lodged in the ampulla could occlude both the common bile duct (CBD) and the pancreatic duct. The obstruction caused the formation of a common channel that would allow
reflux of bile into the pancreatic duct with activation of
pancreatic enzymes and pancreatitis[5,6].
The pathophysiology and treatment of AP has been
intensely studied during the last century and our aim is to review recent evidence and achievements in the diagnosis and treatment of this serious condition at the beginning of the 21st century.
EPIDEMIOLOGY
AP is a growing problem in Europe, posing significant
medical, surgical and financial sequelae[7]. A recent
systematic review presented trends in incidence of
the first attack of AP using data from 12 longitudinal
studies[8]. The mean age of the first attack was in the 6th
incidence of gallstone pancreatitis among white women over the age of 60 years[9,10]. The most common causes
were gallstones (11%-56%), idiopathic (8%-44%) and alcohol (3%-66%). However, occult microlithiasis is
probably responsible for most cases of idiopathic AP[11]. Gallstone AP was found to be more common in
female subjects, alcohol pancreatitis more common amongst men and idiopathic pancreatitis similar in both sexes.
The incidence of AP has been reported to be
markedly increasing[8,12,13]. The explanation of this
increased incidence could be explained by the routine testing of pancreatic enzymes in patients presenting with abdominal pain at emergency departments, and in over-diagnosis in cases of non-specific increases in enzymes due to other causes. Another explanation is an increase in the incidence of gallstone disease and obesity in the population.
Although fatalities associated with AP have decreased
over time from 15%-20% to below 5%, the population mortality rate has remained unchanged with increasing age associated with higher mortality[14-16]. A correlation
with duration of disease was also shown with 65% of the
deaths occurring in the first 14 d and 80% within 30 d[17]. Overall, severe AP (SAP) occurs in 10%-20% of
patients and despite improvements in critical care
between 10% and 25% of patients with SAP die[18,19].
PATHOPHYSIOLOGY
The pathogenesis of AP is caused by an inappropriate
activation of trypsinogen to trypsin. Once activated these enzymes are responsible of autodigestion of pancreatic tissues resulting in necrosis of the acini and pancreatic islets with interstitial fat necrosis and necrotizing vasculitis[20].
These pathological changes in the pancreatic gland are responsible for releasing active pancreatic enzymes into the bloodstream and stimulating the production of inflammatory cytokines such as interleukin-1, interleukin-6 and interleukin-8 from neutrophils, macrophages and lymphocytes. The release of those interleukins and tumor necrosis factor-α (TNF-α) from macrophages triggers an inflammatory cascade which
leads to the systemic inflammatory response syndrome (SIRS)[21]. SIRS may develop into acute respiratory
distress syndrome or multiorgan dysfunction syndrome.
This systemic inflammatory response to pancreatic injury marks the “first or early phase” of the natural course of SAP, which normally characterizes the first 14 d of the
disease[21,22]. In this phase, organ failure is common and
often is not associated with infection[23]. The “second
or late phase” which starts 14 d after the onset of the disease, is marked by infection of the gland, necrosis
and septic systemic complications causing a significant
increase in mortality[24]. Infection of the necrotic
pancreas occurs in the 8% to 12% of the patients with
AP and in 30% to 40% of patients with necrotizing
pancreatitis, and it is considered the most important risk factor of necrotic pancreatitis[25,26].
DIAGNOSIS
Abdominal pain together with elevation of plasma levels of pancreatic enzymes is the cornerstone of diagnosis. The pain normally is generalized in the upper abdomen and occurs suddenly without a prodrome. The pain, which tends to last a few days, is often radiated in a bandlike manner to the lower thoracic region of the back. Nausea and vomiting normally appear in about 90%
of patients and can be severe. Physical signs of severe
disease such as ecchymoses in the flank (Gray-Turner’s sign) or in the periumbilical region (Cullen sign) occurs in less than 3% of patients, and have been associated with a mortality of 37%[27]. Pancreatic enzymes are released into
the circulation during an acute attack. Levels peak early, and decline over 3-4 d. As a consequence, the diagnosis should not rely on arbitrary levels 3 or 4 times greater than normal, but levels should be interpreted in light of the time since the onset of abdominal pain.
Enzymes released by acinar cells during an attack of AP
are amylase and lipase, and their concentration in the serum
is used to confirm the diagnosis[28]. The half-life of elevated
amylase is shorter than that of lipase: the diagnosis using
plasma lipase has slightly superior sensitivity and specificity
and greater overall accuracy than amylase.
There are more specific tests in detection of AP, such as urinary trypsinogen activation peptide (TAP), serum
and urinary trypsinogen[29-32], but these are less widely
available.
Other laboratory tests can exclude metabolic causes such as hypercalcemia and lipid disorders.
AETIOLOGY
In order to optimize the instant management and prevent
recurrence of AP it is essential identify the aetiology.
In the Western world, biliary tract disease (38%) and alcoholism (36%) are accountable for the majority
of cases of AP[33]. However, in up to 10% of cases, the cause of AP remains unknown (idiopathic AP).
Gallstones
In patients with no history of alcohol consumption, an increased level of serum alanine aminotransferase up to 3 times its normal value is indicative of gallstone pancreatitis.
Gallstone pancreatitis, in most cases, is caused by gallstones passing into the bile duct and temporarily lodging at the sphincter of Oddi. However, duct obstruction can also be localized in the pancreatic duct. Although not completely proven, it is thought that duct obstruction leads to increased pancreatic duct pressure with subsequence injury to acinar cells and activation of digestive enzymes. It is supposed that only gallstones with a diameter up to 5 mm can migrate distally into the biliary duct whilst gallstones with a diameter of 8 mm or more remain in the gallbladder[34].
However, a biliary aetiology should not be excluded when liver function tests are normal since 15%-20% of
patients with biliary AP can have normal concentrations
In cases where a biliary aetiology is suspected, the first line of investigation should be a trans-abdominal
ultrasound (T-A US). The value of US lies in its ability
to demonstrate gallbladder stones and dilatation of the CBD as well as other pathology unrelated to the pancreas. In the case of high clinical suspicion of a biliary
cause of AP with normal T-A US, magnetic resonance cholangiopancreatography or endoscopic US should
be performed in order to visualize the presence of microlithiasis or other causes of duct obstruction.
Alcohol
Alcohol consumption is the second cause of AP.
Although the acinar cell is considered the main target of damage by ethanol, there is not an accepted explanation for why some patients are more predisposed to developing
AP than others who consume similar quantities of
alcohol. The pathogenesis of alcohol pancreatitis can be explained by a combination of environmental and genetic factors. Genetic studies have suggested that, in hereditary pancreatitis, mutation of the cationic trypsinogen gene
and serine peptidase inhibitor, Kazal type 1 (SPINK1) genes can promote AP in the presence of alcohol[36].
Post-ERCP acute pancreatitis
The risk of developing AP after endoscopic retrograde cholangiopancreatography (ERCP) is around 5%[37]. The main risk factors for post-ERCP AP include female
gender, presence of periampullary diverticulum, and procedure-related factors such as a cannulation time of more than 10 min and major papilla sphincterotomy. However, the risk of developing asymptomatic hyper-amylasemia, which appears in 35%-70% of patients, seems to be linked with procedure-related factors[38].
Trauma
Abdominal trauma causes an elevation of amylase and
lipase levels in 17% of cases and clinical AP in 5% of cases. Pancreatic injury occurs more often in penetrating
injuries (e.g. from knives, bullets) than in blunt abdominal trauma (e.g. from steering wheels, horses, bicycles). Blunt injury may crush the gland across the spine, leading to a ductal injury in that location[39].
Drug-induced pancreatitis
Drug-induced pancreatitis is considered a rare event (0.1%-2%) and is normally mild and self-limiting. In the
literature, the true incidence of drug-induced AP is not
known since the evidence is derived mainly from case reports and the diagnosis is always challenging because of the difficulty in distinguishing the effects of drugs
from other causes of AP[40].
Dr ugs strongly associated with AP include
azathioprine, sulfonamides, sulindac, tetracycline, valproic acid, didanosine, methyldopa, estrogens, furosemide, 6-mercaptopurine, pentamidine, 5-aminosalicylic acid compounds, corticosteroids, and octreotide.
Infections
Infections are accountable for less than 1% of all AP
and tend to be milder than biliary and alcohol-induced
AP[41]. Viral infections such as Epstein-Barr, coxsackie
virus, echovirus, varicella-zoster and measles are the most
common causes of infectious AP especially in children.
Bacterial causes include Mycoplasma pneumoniae,
Salmonella typhosa, Leptospira, Campylobacter and Mycobacterium tuberculosis.
Worldwide, ascariasis can cause AP by migration of
worms in and out of the duodenal papillae.
Hereditary pancreatitis
Hereditary pancreatitis is an autosomal dominant gain-of-function disorder related to mutations of the cationic trypsinogen gene (PRSS1), which has an 80% penetrance. Mutations in this gene cause premature conversion of trypsinogen to active trypsin causing pancreatic autodigestion. This genetic syndrome is associated with a high risk of developing chronic pancreatitis at a young age and of developing pancreatic cancer[42].
Mutations in the SPINK1 gene, which blocks the
active binding site of trypsin, rendering it inactive, are
associated with acute and chronic pancreatitis. Patients who have severe SPINK1 mutations normally develop
chronic pancreatitis in childhood[43].
In patients with mild CFTR gene mutations, an increased risk of developing acute and chronic pancreatitis has been observed in comparison with the general population.
Hypercalcemia
Hypercalcemia and primary hyperparathyroidism can
lead to AP. Hypercalcemia, which causes less than 1% of
all cases of pancreatitis, normally appears with excessive doses of vitamin D, familial hypocalciuric hypercalcemia and total parenteral nutrition.
Hypertriglyceridemia
AP usually does not occur until serum triglyceride levels
reach 1000 mg/dL. Hypertriglyceridemia causes about 2%
of AP and it is normally associated with type Ⅰ, Type Ⅱ
and Type Ⅴ hyperlipidemia. The triglyceride level should
be measured as soon as clinical presentation of AP appears
since this level tends to decline during hospitalization because of fasting and Ⅳ fluid resuscitation.
Acquired hypertriglyceridemia can appear in adults because of alcoholism, obesity and poorly controlled diabetes mellitus.
In order to prevent recurrent attacks of AP, the
patient should be placed on a low-fat diet, a regular exercise regimen, and tight control of diabetes, with use of lipid-lowering drugs such as statins.
Developmental abnormalities of the pancreas
The pancreas develops from two buds stemming from the alimentary tract of the developing embryo.
Pancreas divisum is a failure of the dorsal and ventral
pancreatic ducts to fuse during embryogenesis and it occurs
in about 5%-7% of the healthy population. Pancreatitis
by a narrow duct at its papillary origin.
Sphincter of Oddi dysfunction (SOD) is suspected
clinically by recurrent episodes of epigastric or right upper quadrant pain that last 30 min or longer and that are not relieved by bowel movements or by antiacids[44]. SOD can lead to AP by causing increased pancreatic ductal pressure. However, SOD is a controversial cause of AP especially in patients without elevated sphincter
pressures on manometry.
Tumor
Obstruction of the pancreatic ductal system by a tumor
can increase the intraduct pressure and causing AP in
proximately 14% of patients suffering from pancreatic tumors.
Pancreatic ductal carcinoma, ampullary carcinoma,
islet cell tumor, solid pseudotumor of the pancreas, sarcoma, lymphoma, cholangiocarcinoma, or metastatic
tumor can cause AP.
In addition, a pancreatic cystic neoplasm, such
as intraductal papillary-mucinous neoplasm (IPMN),
mucinous cystadenoma, or serous cystadenoma, can also
cause AP[45].
Postoperative
AP may occur in the postoperative period of various
surgical procedures. The mechanism of this pancreatitis includes transient intraoperative hypotension or pancreatic
trauma by intraoperative manipulation. Postoperative AP is often a difficult diagnosis to confirm, and it has a higher
complication rate than pancreatitis associated with other etiologies.
Autoimmune pancreatitis
This relatively newly described entity is an extremely rare
cause of AP. The diagnosis of autoimmune pancreatitis
has to be confirmed by specific radiological and
histological findings. Radiologically, there is a focal mass in
the pancreatic head on computed tomography (CT) scan and irregular narrowing of the proximal pancreatic duct
on ERCP.
Patients normally have an elevated Ig G4 level in the
serum and infiltration of IgG4-containing plasma cells in the pancreas. It normally occurs in young people who also suffer from inflammatory bowel disease, primary
sclerosing cholangitis, primary biliary cirrhosis and
Sjogren’s syndrome. The therapy of choice is based on
steroids.
PREDICTION OF SEVERITY
Although, the majority of patients have a mild episode of
AP, it is difficult to identify the patients who are at risk of
developing severe disease on admission to the hospital. There is agreement that there is still a need for an early objective measure of severity. Clinical examination in the first 24 h of admission although specific, lacks sensitivity and hence is unreliable and should be supported by objective measures[46].
Although there is no ideal single serum marker for pre-dicting severity, C-reactive protein (cut-off of 150 mg/L) is a useful indicator of necrosis with a sensitivity and
specificity of 80% but is required to be measured more
than 48 h after the onset of symptoms[47-49].
Different markers of severity shown to be useful
on admission are serum procalcitonin and urinary TAP
and trypsinogen-2[50], serum interleukins-6 and -8 and
polymorphonuclear elastase at 24 h after admission[51].
The severity of the inflammatory response to pancreatic injury and the presence of organ failure are normally assessed by scoring systems. Multi-factorial
scoring systems based on clinical and laboratory findings such as the Ranson’s score, Glasgow Score and the Acute Physiology and Chronic Health Evaluation Ⅱ (APACHE Ⅱ) are considered accurate predictors of the
severity of AP[52,53].
In 1974, John Ranson selected 11 prognostic signs based on statistical analysis of 43 parameters gathered
retrospectively from 450 AP patients. Five of these
criteria are measured on admission and the remaining 11 are measured 48 h post admission (Table 1).
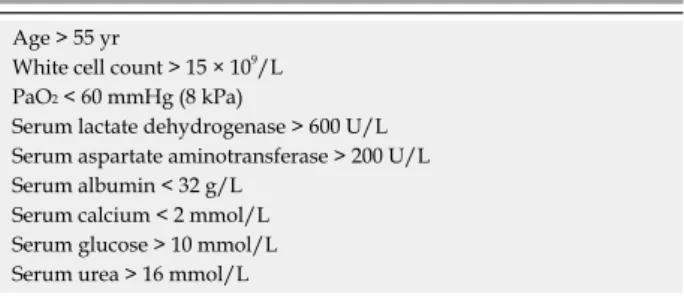
The Glasgow severity scoring system considered 9 variables and can be applied from admission although it is not complete for 48 h[54] (Table 2).
Developed in 1985, the APACHE Ⅱ score has been
used to predict severity in AP patients[55]. APACHE Ⅱ
measures 12 physiological variables and additional points are added based on patient age and on severe baseline
chronic diseases. Measuring APACHE Ⅱ score daily can allow an assessment of progression of the disease[56].
Other markers should also be considered such as body mass index (BMI) since obesity has been shown to increase Table 1 Ranson’s criteria for prediction of severity of acute
pancreatitis
On admission During initial 48 h
Age > 55 yr Hemoglobin falls below
10 mg/dL
White cell count < 16 × 109/L Blood urea nitrogen increases by > 5 mg/dL
Lactate dehydrogenase > 350 U/L Calcium < 8 mg/dL Aspartate aminotransferase
> 250 U/L
PaO2 < 60 mmHg (8 kPa)
Glucose > 200 mg/dL Base deficit > 4 mEq/L Fluid sequestration > 6 L
Table 2 Glasgow (Imrie) severity scoring system for acute pancreatitis
Age > 55 yr
White cell count > 15 × 109/L PaO2 < 60 mmHg (8 kPa)
Serum lactate dehydrogenase > 600 U/L Serum aspartate aminotransferase > 200 U/L Serum albumin < 32 g/L
Serum calcium < 2 mmol/L Serum glucose > 10 mmol/L Serum urea > 16 mmol/L
the risk of systemic complications and mortality[57]. This has led to a modification of the APACHE Ⅱ scoring system which includes up to 2 points for obesity[58]. This system is called APACHE-O and although Johnson and
colleagues have reported its superiority in predicting outcomes, other authors have not confirmed these results[59].
Although these scoring systems can help the
physician in a first assessment of the patient, the most
important distinction in terms of prediction of severity is the presence of severe manifestations of the disease
such as evidence of SIRS and presence of organ failure. Mofidi, in a recent retrospective study of 259 patients admitted with AP, showed that the mortality rate was
significantly higher in patients who developed or had
persistent SIRS at 48 h after admission (25.4%) than in patients who had transient SIRS (8%) or no SIRS in the first 48 h (0.7%)[60].
Therefore, immediate assessment should include clinical evaluation particularly of any cardiovascular, respiratory and renal compromise, BMI, chest X-ray and different acute diseases scores. The presence of any single and/or multiple organ failure has been increasingly recognized as an important variable for predicting
mortality from AP. The most common organ dysfunction
scores used for critically ill patients are the Multiple Organ
Dysfunction Score (MODS) and the Sequential Organ Failure Assessment (SOFA)[61,62] (Tables 3 and 4).
Since the SOFA score uses the mean arterial pressure
and therapeutic interventions with vasopressors, its outcome prediction for cardiovascular dysfunction is
considered better than the MODS[63].
Since the mortality in the presence of pancreatic
necrosis increases from 1% to 10%-23%, the importance of early detection of pancreatic necrosis is not to be overlooked[64]. Contrast-enhanced CT has been considered
the “gold standard” for the diagnosis of pancreatic necrosis[65,66].
However, it is not clear how soon the full extent of the necrotic process occurs, but it is at least 4 d after onset of the symptoms, and early CT may therefore underestimate the final severity of the disease. Finally, unless some management decision is required based on the extent of
necrosis (for example use of prophylactic antibiotics), CT for staging is unlikely to materially affect the management
of patients with AP during the first week of the illness. If CT staging of AP is required, the CT severity index (CTSI)
as proposed by Balthazar should be used (Table 5). Recent
studies demonstrated that AP patients with a CTSI higher
than 5 had 8 times higher mortality. Moreover, they were 17 times more likely to have a prolonged hospital course and were 10 times more likely to require necrosectomy than their counterparts with CT scores < 5[67]. There
is also evidence that the site of pancreatic necrosis is an important prognostic factor with a worse outcome observed in patients with necrosis affecting the head of the pancreas[68]. The finding of free intraperitoneal fluid
and extensive peri-pancreatic fat stranding have also been demonstrated to be associated with worse outcomes.
Although CT is useful in detecting pancreatic necrosis, it is not able to detect a super-infection of necrosis in the later stage of the disease unless gas bubbles are seen within the necrotic area[69].
Patients with persisting organ failure, or in whom
new organ failure develops, and in those with persisting pain and signs of sepsis, will require evaluation by dynamic contrast enhanced CT. CT evidence of necrosis correlates well with the risk of other local and systemic complications.
Since pancreatic necrosis commonly remains stable
in appearance, a follow-up CT scan at 3 to 4 wk is not normally considered[70].
MANAGEMENT OF MILD AP
In most cases AP is mild and its initial management
is directed towards maintenance of adequate organ perfusion in order to reduce the systemic complications caused by the pancreatic injury. This consists of fluid resuscitation, analgesia, oxygen administration, antiemetics and repeated evaluation of the patient’s vital signs with the intention of identifying early manifestations of organ dysfunction.
Since fluid loss in mild pancreatitis can be significant,
appropriate fluid resuscitation is a crucial part of the management in order to improve the microcirculation of the pancreas (Figure 1).
As a marker of third-space losses, hemoconcentration has been associated with a higher probability of developing pancreatic necrosis and organ failure[71].
Moreover, patients who experienced a worsening or a lack of improvement of their hemoconcentration after 24 h
of fluid resuscitation have a higher chance of developing
necrotizing pancreatitis[72]. Therefore, all patients with AP should receive very close clinical monitoring with
emphasis on vigorous fluid resuscitation. Rehydratation has to be monitored with the help of invasive monitoring such as a Foley catheter and a central line to measure the urine output and central venous pressure, respectively.
Patients who have borderline cardiac dysfunction or respiratory failure may require a Swann-Ganz catheter.
Crystalloids are preferred to colloids in most instances.
However, electrolyte disturbances and fluid overload can Table 3 Modified multiple organ dysfunction score
Organ system involved Score
1 2 3 4 5
Cardiovascular PAHR (beats/min)
≤ 10 10-15 30-15 20-30 > 30
Respiratory PaO2/FiO2 (mmHg)
> 300 300-225 150-225 75-150 < 75
Renal creatinine (μmol/L) < 100 100-200 200-350 350-500 > 500 Neurological glasgow coma
score
15 14-13 12-10 9-7 ≤ 6
Hematological platelet
count (× 109/L) > 120 80-120 50-80 20-50
≤ 20
Hepatic bilirubin (μmol/L) < 20 20-60 60-120 120-240 > 240
be serious complications of fluid resuscitation especially in
patients who have developed cardiovascular dysfunction or acute respiratory distress syndrome.
Adequate pain control is essential. Parenteral analgesia
is usually needed with an advantage in using patient-controlled analgesia. Opiates are normally used including
morphine and meperidine. Since there are no studies
directly comparing the effects of meperidine or morphine on sphincter of Oddi manometry, morphine seems to
provide more benefit by offering a longer duration of action and fewer side effects[73].
Supplemental oxygen is required in all patients. The
oxygen saturation should be maintained at 95% or higher with supplemental oxygen administered by nasal cannula or by a face mask in order to prevent pancreatic necrosis.
The role of activated protease in producing organ failure is not clear. Although antiprotease treatment has been successful in experimental pancreatitis, it has not been
shown to offer a survival benefit, but only a reduction of
the incidence of complications in human disease[74,75]. Since cytokines could play an important role in AP,
many agents have been used in experimental animals to ameliorate or prevent the inappropriate activation of the immune systems.
Anti-TNF-α antibody has been shown to reduce the
induction of further cytokines by inflammatory cells but
the peak TNF-α production appears to be within 1 or 2 h of the onset of disease. This action can compromise the use of TNF-α blockers in the clinical setting since the presentation and diagnosis of disease occurs after the peak of TNF-α production[76-78].
The role of calcium channel signaling in cytokine
release and systemic organ injury in AP has been reported
in one animal study. In this study, Hughes demonstrated that calcium channel blockers were associated with a dramatic reduction of TNF-α release with an improvement in overall survival from 40% to 80% between untreated rats and animals pre-treated with diltiazem, respectively[79].
An interleukin-1 receptor antagonist has been shown to be associated with decreased severity of pancreatitis in animal models, however these results have not been translated into clinical practice so far[80,81].
Table 4 Sequential organ failure assessment score (SOFA)
Organ system involved Score
1 2 3 4 5
Cardiovascular No
hypotension MAP < 70 mmHg
Dopamine or dobutamine
(any dose)
Dopamine > 5 μg/kg per min or adrenaline (epinephrine) < 0.1 μg/kg per min or noradrenaline (norepinephrine) < 0.1 μg/kg per min
Dopamine < 0.1 μg/kg per min or > 15 μg/kg per min or adrenaline > 0.1 μg/kg per min or noradrenaline > 0.1 μg/kg per min
Respiratory PaO2/FiO2 (mmHg) > 400 400-300 300-200 200-1001 ≤ 1001
Renal creatinine (μmol/L) < 100 100-200 200-350 350-500 > 500
Neurological glasgow coma score 15 14-13 12-10 9-7 ≤ 6
Haematological platelet count (× 109/L) > 150 150-100 100-50 20-50 ≤ 20
Hepatic bilirubin (μmol/L) < 20 20-60 60-120 120-240 > 240
1These values are calculated with ventilatory support. MAP: Mean arterial pressure; SOFA: Sequential organ failure assessment, and is calculated as the sum of the scores for the individual organs.
Table 5 Acute pancreatitis graded with CT and CT severity index table
Grade CT finding Points Necrosis Severity index
Percentage Additional points
A Normal pancreas 0 0 0 0
B Pancreatic enlargement 1 0 0 1
C Pancreatic inflammation and/or peripancreatic fat 2 < 30 2 4
D Single peripancreatic fluid collection 3 30-50 4 7
E Two or more fluid collections and/or retroperitoneal air 4 > 50 6 10
Supportive care investigate pt for
other causes
Non biliary Biliary
Cholangitis, jaundice
Yes No
ERCP + sphincterotomy
Laparoscopic cholecystectomy +
IOC
CBD stone
Yes No
Laparoscopic CBD exploration or
postop ERCP Discharge home Predicted mild
pancreatitis
Since acute lung injury in AP results in an
up-regulation of vascular adhesion molecule-1 (VCAM-1) cell surface receptor expression on pulmonary vascular endothelium, and neutrophil sequestration, experimental studies showed that blocking VCAM-1 decreased lung
injury in AP[82,83]. It is believed that a VCAM-1 antagonist
can offer a therapeutic option to improve the systemic
manifestation and therefore the prognosis of AP.
Administration of monoclonal antibodies directed against the adhesion molecule, junctional adhesion molecule C expressed by endothelial cells showed a significant reduction in secretion of inflammatory cytokines, and in acinar cell necrosis[84].
Recently, there have been reports considering 5-fluorouracil treatment more effective compared to single inflammatory cytokine blockers in modulation
of inflammatory mediators in experimental AP[85].
However, although the last decade has seen an increase in experimental studies on cytokines, the clinical treatment of the disease remains unchanged.
MANAGEMENT OF SAP: “EARLY PHASE”
SAP is defined by the Atlanta classification as an AP
with local and/or systemic complications[86]. Some
patients develop pancreatitis-associated organ failure
during the early phase of the SAP[87].
The initial management of SAP is supportive based on fluid resuscitation, analgesia and enteral nutrition.
All patients should have thrombo-prophylaxis with low molecular weight heparin; however the decision to begin stress ulcer prophylaxis is still debatable.
SAP induces a catabolic state. Hence, early nutritional
support is essential in order to avoid malnutrition.
An important issue in the early treatment of SAP is
the optimal delivery of nutrition. After initial enthusiasm
towards parenteral nutrition (PN), recent guidelines advise early enteral nutrition (EN) through a nasojejunal
tube[88]. Patients with AP are characterized by loss of
the gut barrier function which is involved in both local and systemic infectious complications[89]. In a
meta-analysis analyzing 5 randomized control trials since
1997, Petrov et al[90] showed that EN, compared to PN, had a statistically significant lower risk of infection and
mortality. Although there are studies supporting a lower infection rate when the tube is positioned in the jejunum,
Eatock et al[91] demonstrated that nasogastric feeding is
as good as nasojejunal feeding.
Although the role of probiotics in AP has been
investigated in different clinical trials and meta-analyses, in a recent randomized controlled trial Besselink et al[92] showed that, in patients with predicted SAP, probiotic
prophylaxis did not reduce the risk of infectious complications and was even associated with an increased risk of death.
Although the main management in the early phase
of SAP is advocated to be mainly conservative and
supportive in order to avoid organ dysfunction, there are conditions such as gallstone pancreatitis for which early endoscopic or surgical intervention has to be sought.
The benefits of ERCP with sphincterotomy (ES)
has been studied in 3 randomized trials[93-95] and 2
meta-analyses[96,97]. Patients with predicted mild acute biliary pancreatitis (ABP) in the absence of cholangitis have not shown benefits from an early ERCP.
The decision on management of patients with
predicted severe ABP is still debatable. The most recent
United Kingdom guidelines recommend that urgent
therapeutic ERCP should be performed within 72 h of admission in all patients with predicted severe ABP,
whether or not cholangitis is present[98].
However, a recent meta-analysis by Petrov et al[97] demonstrated that early ERCP with or without ES had
no beneficial effect in patients with predicted mild or
severe ABP without cholangitis. The conclusion of this
study was partially supported by the 2007 guidelines of the American Gastroenterology Association which stated
that early ERCP in patient with severe ABP without
signs of acute cholangitis is still not uniformly accepted in the literature[99].
However, optimal timing of laparoscopic
cholecystec-tomy (LC) in patients with ABP is still contentious. In mild ABP, LC with operative cholangiography has been considered the definitive treatment[100].
Early laparoscopic cholecystectomy (ELC) can be
performed as soon as the serum amylase decreases and symptoms improve[101].
Heinrich et al recently analyzed 4 prospective trials evaluating the optimal timing for surgery and concluded
that ELC should be preferred in patients with mild to moderate ABP while in patients with severe ABP
who did not have surgery for necrotizing pancreatitis, cholecystectomy appears to be favorable after full recovery[101-105].
MANGEMENT OF SAP: “LATE PHASE”
The main life-threatening event which characterizes the
late phase of SAP is infection of the necrotic pancreatic
parenchyma. Infection tends to occur in 10% to 50% of patients with necrotizing pancreatitis and develops 2-3 wk after the onset of symptoms[26,106-108]. The mortality
increases from 5%-25% in patients with sterile necrosis to 15%-28% when infection occurs[24,107,109-112].
The infection is thought to be caused by translocation of enteric flora from the small and large bowel, since gram-negative bacteria such as Esherichia coli has been the most common species isolated. The use of antibiotic prophylaxis has changed the microbiology in favor of gram-positive and fungal organisms such as Staphylococcus
species and Candida[113-116].
The debate on prophylactic antibiotics in sterile
necrotic pancreatitis is still open. Since 1991, when
Bradley introduced the concept of conservative treatment of sterile necrotic pancreatitis with the use of antibiotics, different randomized, controlled trials were
conducted in order to investigate the beneficial effects
of antibiotic therapy[117]. Initial studies were conducted with imipenem, ciprofloxacin and metronidazole[118-121].
SAP is capable of reducing the incidence of bacterial
infection of pancreatic necrosis but it was unable to improve hospital mortality.
Two placebo-controlled, double-blind trials by Isenmann and Dellinger demonstrated a lack of
significant benefit of prophylactic antibiotics on infection
and surgical intervention rates[122,123]. However, a recent
Cochrane meta-analysis, which included the Isenmann study, showed a reduction in mortality with the use of β-lactams, although there was no evidence of a reduction in the pancreatic necrosis infection rate[124].
Despite the requirement for further multicenter double-blind studies, the use of prophylactic antibiotics in patients with proven necrotic pancreatitis on CT has been advocated. β-lactam agents are preferred to quinolones and the length of the therapy has to be at least 2 wk (Figure 2).
SURGICAL INTERVENTION
Surgery is considered the gold standard treatment for
proven infected pancreatic necrosis[125].
Since Bradley has introduced the concept of
conservative treatment in non-infected pancreatic necrosis, the timing of surgical intervention has changed substantially[117]. In the past, early surgical intervention
was advocated and had a mortality rate up to 65%[126,127]. Recent studies demonstrated the benefit of postponing
surgical intervention in reducing the mortality rate to 27%, allowing the immune system to demarcate the pancreatic necrosis[128,129].
The IAP (International Association of Pancreatology)
Guidelines recommend that a patient with infected necrotic pancreatitis has to undergo surgery in the 3rd or 4th wk after the onset of symptoms[88]. However,
postponing surgical intervention in necrotic pancreatitis can lead to prolonged use of antibiotics and an increased antibiotic resistance and higher incidence of Candida
infection[130,131].
In a recent retrospective study, Besselink strongly advised avoidance of surgical intervention in the first 14 d even in the presence of multiple organ failure, and withholding of necrosectomy until day 30[132].
The aim of surgical management is to remove all the pancreatic tissue with necrosis in order to reduce the release of inflammatory mediators. Necrosectomy and drainage of infected acute necrotizing pancreatitis can be performed with different approaches such as radiological, endoscopical and surgical intervention.
Radiologic necrosectomy
Since Freeny presented the first series of patients with
infected necrotic pancreatitis treated with only CT-guided percutaneous catheter drainage in 1998, this approach has not been considered very successful in debriding thick necrotic pancreatic tissue[133-135]. However,
a combination of percutaneous catheter drainage and intensive care support can offer an alternative to surgery[136]. In unstable patients, percutaneous catheter
drainage can be successful in draining pus and reducing the systemic manifestations of sepsis and in doing so, prepare the patient for surgery[133].
The placement of the catheter can be obtained through an anterior or retroperitoneal approach. To achieve an adequate drainage of the thick pancreatic necrotic collection, catheters with multiple side holes with a minimum diameter of 12-14 Fr are required[137]. Percutaneous drainage can lead to suprainfection of
the pancreatic collection due to colonization of the indwelling catheter. However, clinical infection has been shown to be unlikely if all material is drained within 2-3 d.
Endoscopic necrosectomy
The endoscopic approach with the help of endoscopic
US (EUS) has been reported to be effective in recent
retrospective studies[138-141]. This technique can be considered as a natural orifice transluminal endoscopic surgery (NOTES) procedure since it employs a
transenteric access route.
The most common access is through the posterior
wall of the stomach under EUS and under Doppler
imaging in order to avoid vascularized areas. The procedure is normally performed under sedation and requires good endoscopic skills.
Initially, a trans-gastric puncture under EUS guidance
is performed using a 19-gauge needle through which a guidewire is passed into the cavity and coiled multiple times under fluoroscopic vision. The tract is dilated by a dilator which is passed over the guide and further dilatations can be done using 10-15 mm balloons. This dilation will allow an optimal insertion of the upper endoscope into the pancreatic cavity. The necrosectomy
Predicted severe pancreatitis
Biliary Non biliary
Urgent ERCP + ES within 72 h if signs
of cholangitis
Refer to ITU/HDU daily MODS/SOFA score CECT abdomen day 610
> 30% FNA proven infected necrosis
Start antibiotics, consider surgical debridement 4 wk after
onset of symptoms
Observe
Pt stable Deterioration
Urgent laparoscopic cholecystectomy if biliary aetiology
(within 6 wk)
Repeat CECT with FNA if necrosis is present
Yes No
is performed using different devices such as polypectomy snare, transparent cap baskets and different graspers.
The debridement of necrotic tissue is followed by copious irrigation with normal saline solution. A pig-tail drain inserted into the cavity and a nasocystic drain are used for continuous lavage. The lavage has to be repeated until all the necrotic material is removed[139,140].
The use of antibiotics is advised until the nasocystic drain has been removed.
Although optimal necrosectomy is normally achieved with multiple sessions, in a recent retrospective study which included 6 consecutive patients, Mathew
et al[139] demonstrated that complete removal of necrotic
tissue can be achieved with a single procedure. In this study none of the patients had procedure-related complications; however injury to visceral and vascular structures must be borne in mind as potential life-threatening complications.
Recent data from the literature indicated that the endoscopic approach could be a safe and effective modality for management of infected pancreatic necrosis with potentially lower morbidity than the traditional surgical approach. However, the data was from small prospective studies and multicenter prospective randomized trials are needed to clarify the role of the endoscopic approach in necrotic pancreatitis.
Surgical necrosectomy
The aim of surgery is to control the focus of infection through removal of necrotic tissue. This is based on preserving the endocrine and exocrine functions of the pancreas and allowing postoperative removal of retroperitoneal debris and exudates.
Until recently, necrosectomy was generally performed only by an open route. In order to achieve postoperative continuous drainage of debris, 4 different techniques have been advocated[142]: (1) open necrosectomy with
open packing and planned re-laparotomy[143-149]; (2)
open necrosectomy with planned re-laparotomy, staged and repeated lavage[150-153]; (3) open necrosectomy with
continuous lavage of the lesser sac and retroperito-neum[153-161]; (4) open necrosectomy with closed packing[162].
Open necrosectomy with open packing and planned re-laparotomy: The abdominal cavity is filled with non-adherent packing. Successive laparotomies are performed
every 48 h for further debridement which can be performed in intensive care under sedation. The abdomen is closed with drains when granulation tissue appears.
Open necrosectomy with planned re-laparotomy, staged and repeated lavage: After a primary necro-sectomy repeated debridement is performed on alternate days until all necrotic tissue has been replaced by granulation tissue. To improve the surgical access some surgeons use an abdominal wall zip.
Open necrosectomy with continuous lavage of the lesser sac and retroperitoneum: Primary
necro-sectomy is followed by continuous closed lavage of the
retroperitoneum. The procedure is based on insertion of 2 or more double 20-24 French drains and a single lumen 28-38 French silicone rubber on each side of the abdomen and placed with the tip at the tail of the pancreas. The smaller lumen of the drains is used for the
inflow of the lavage and the larger lumen for the outflow.
At least 35-50 L lavage are requested in the first days. Drains can be removed after 2-3 wk.
Open necrosectomy with closed packing: After the
removal of the necrotic tissue, the residual cavity is filled with gauze-packed Penrose drains and suction drains.
Drains are removed after 7 d.
Of these techniques open necrosectomy with closed continuous lavage of the lesser sac and the retroperitoneum seems to have the lowest mortality and it is the most common approach used by surgeons.
In specialized centers, open surgical management of infected necrosis can reduced the mortality from 80% to 10%-20%. This high mortality has induced surgeons to
find a new, less invasive approach in order to reduce the activation of an inflammatory response in patients who
are already seriously ill.
MINIMAL INVASIVE SURGICAL
APPROACH
Over the last 2 decades, the role of minimal invasive surgical approaches to necrotic pancreatitis has increased. Minimal invasive techniques can be classified under 2 groups: (1) video-assisted retroperitoneal debridement (VARD) and (2) laparoscopic transperitoneal debridement
(LTPD).
VARD
This approach was first described by Carter et al[163] in
2000 using an operative nephroscope inserted over an 8
Fr pigtail catheter, positioned under CT guidance. Since
this initial experience, different studies have performed necrosectomy through a retroperitoneal approach using a rigid nephroscope or zero degree laparoscope.
This technique is performed by placing the patient in a supine position with his left flank elevated. A subcostal or intercostal incision is made to follow the retroperitoneal drains up to the necrotic area which is opened bluntly through digital examination. Necrotic tissue is debrided by a ring forceps and a suction device. A 10 mm laparoscope and a long 10 mm trocar are inserted through the retroperitoneal wound. Under laparoscopic vision, the remaining necrotic tissue is removed by laparoscopic graspers. Two surgical drains are inserted into the necrotic cavity via the incision[164-170].
Recently, Bucher et al[170] described a new technique
based on a single laparoscopic port in order to avoid repeated necrosectomies. Using a 5 mm laparoscope with a 30 degree optic, a 12 mm trocar was placed in the drain tract. Through this big trocar, 5 mm instruments were used simultaneously with the 5 mm laparoscope. The
pressure of 8 mmHg in order to avoid potential bacterial translocation. At the end the procedure, the cavity was inspected with a 30 degree 10 mm laparoscope. Among 8 patients who underwent this new procedure only one did not have a successful debridement and had repeated necrosectomy.
The VARD approach has the great advantage of avoiding peritoneal contamination but is limited in necrosis extraction, and the need for repeated sessions is quite common. Other limitations of VARD are its low ability in detecting colonic ischemia, and in performing cholecystectomy or insertion of a feeding jejunal tube at the time of debridement[141].
LTPD
Cuschieri described for the first time the technique of laparoscopic infracolic necrosectomy with irrigation of the lesser sac as a valid alternative to open necrosectomy[171].
Although this laparoscopic technique has been described in the literature through case reports since 2002[172-175], only recently Parehk reported a retrospective
study on hand-assisted laparoscopic surgery for pancreatic necrosectomy[174]. This study described 18
patients with necrotizing pancreatitis who underwent laparoscopic necrosectomy using an infracolic approach to access the lesser sac with a hand access port in order to enlarge the window in the transverse mesocolon and to bluntly remove the necrotic tissue. The outcomes were encouraging with mean length of stay of 16.3 d after the procedure and a reduction in the incidence of major wound complications.
This technique gives a better exposure of the lesser sac, left paracolic gutter and head of the pancreas, apparently overcoming the main limitation of the retroperitoneal approach in not debriding necrotic tissue completely. On the other hand the transperitoneal approach carries the risk of peritoneal contamination with infected necrosis.
Despite the use of less invasive techniques, complications following debridement of necrotic
pancreatic tissue are still common. Pancreatic or
enterocutaneous fistulae occur in 30% of patients and it seems related to the severity and extent of the underline necrosis[176]. Fistulae should be managed
conservatively initially, deferring surgical closure of
fistulae until pancreatitis is completely resolved. Other
early complications are wound infection and wound dehiscence which seems less common with the minimal
invasive approach. Postoperative bleeding is normally
treated through an endovascular procedure with the help of an interventional radiologist.
Late complications such as pancreatic insufficiency and development of organized sterile necrosis are also common[177].
CONCLUSION
Although most patients with AP will have a benign
outcome, it is crucial to assess the patient using multi-factorial scoring systems and inflammatory markers in
order to evaluate the severity of the condition.
Early management should be conservative, based on fluid resuscitation with a focus on maintaining optimal
organ perfusion. Managing the patient in the ICU should be contemplated when patients show signs of clinical deterioration.
A CT scan should be performed at least 48 h after the onset of symptoms and is considered the gold standard for diagnosis of necrotic pancreatitis. As part of the conservative intensive treatment, nasojejunal feeding is recommended in order to reduce bacterial translocation.
In ABP, early ERCP in patients without signs
of acute cholangitis is not recommended. Infected necrotizing pancreatitis is the main indication for surgical debridement. The best time of surgery is 3-4 wk after the onset of the condition.
The last 2 decades have seen the emergence of new minimal invasive approaches in performing surgical debridement. However, no randomized controlled studies have been published in comparing different techniques.
Nevertheless, the results of the PANTER trial,
a prospective multi-institutional randomized study comparing VARD versus open laparotomy, are still awaited[178].
REFERENCES
1 Sbarounis CN. Did Alexander the Great die of acute pancreatitis? J Clin Gastroenterol 1997; 24: 294-296
2 Bernard C. Leçons de physiologie expérimentale appliquée à la médecine. 2 vols. Paris: Baillière, 1855-1856
3 Fitz RH. Acute Pancreatitis: a consideration of pancreatic
hemorrhage, hemorrhagic, suppurative and gangrenous
pancreatitis of disseminated fat necrosis. Boston Med Surg J
1889; 120: 181-235
4 Chiari H. Über selbstverdauung des menschlichen Pancreas.
Z Heilk 1896; 17: 69-96
5 Opie EL. The etiology of acute hemorrhagic pancreatitis.
Johns Hopks Hosp Bull 1901; 12: 182-188
6 O'Reilly DA, Kingsnorth AN. A brief history of pancreatitis.
J R Soc Med 2001; 94: 130-132
7 Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut
1998; 42: 886-891
8 Yadav D, Lowenfels AB. Trends in the epidemiology of
the first attack of acute pancreatitis: a systematic review.
Pancreas 2006; 33: 323-330
9 Chwistek M, Roberts I, Amoateng-Adjepong Y. Gallstone
pancreatitis: a community teaching hospital experience. J Clin Gastroenterol 2001; 33: 41-44
10 Lévy P, Boruchowicz A, Hastier P, Pariente A, Thévenot T, Frossard JL, Buscail L, Mauvais F, Duchmann JC, Courrier A, Bulois P, Gineston JL, Barthet M, Licht H, O'Toole D, Ruszniewski P. Diagnostic criteria in predicting a biliary
origin of acute pancreatitis in the era of endoscopic ultrasound: multicentre prospective evaluation of 213 patients. Pancreatology 2005; 5: 450-456
11 Cavallini G, Frulloni L, Bassi C, Gabbrielli A, Castoldi L, Costamagna G, De Rai P, Di Carlo V, Falconi M, Pezzilli R, Uomo G. Prospective multicentre survey on acute
pancreatitis in Italy (ProInf-AISP): results on 1005 patients.
Dig Liver Dis 2004; 36: 205-211
13 Lindkvist B, Appelros S, Manjer J, Borgström A. Trends in
incidence of acute pancreatitis in a Swedish population: is there really an increase? Clin Gastroenterol Hepatol 2004; 2: 831-837
14 Corfield AP, Cooper MJ, Williamson RC. Acute pancreatitis:
a lethal disease of increasing incidence. Gut 1985; 26: 724-729 15 Trapnell JE, Duncan EH. Patterns of incidence in acute
pancreatitis. Br Med J 1975; 2: 179-183
16 Eland IA, Sturkenboom MC, van der Lei J, Wilson JH,
Stricker BH. Incidence of acute pancreatitis. Scand J Gastroenterol 2002; 37: 124
17 Floyd A, Pedersen L, Nielsen GL, Thorladcius-Ussing O,
Sorensen HT. Secular trends in incidence and 30-day case
fatality of acute pancreatitis in North Jutland County,
Denmark: a register-based study from 1981-2000. Scand J Gastroenterol 2002; 37: 1461-1465
18 McKay CJ, Imrie CW. The continuing challenge of early
mortality in acute pancreatitis. Br J Surg 2004; 91: 1243-1244 19 Gloor B, Müller CA, Worni M, Martignoni ME, Uhl W,
Büchler MW. Late mortality in patients with severe acute
pancreatitis. Br J Surg 2001; 88: 975-979
20 Hirano T, Manabe T. A possible mechanism for gallstone
pancreatitis: repeated short-term pancreaticobiliary duct obstruction with exocrine stimulation in rats. Proc Soc Exp Biol Med 1993; 202: 246-252
21 Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg 1998; 175: 76-83
22 Gloor B, Reber HA. Effects of cytokines and other inflammatory mediators on human acute pancreatitis. J Int Care Med 1998; 13: 305-312
23 Tenner S, Sica G, Hughes M, Noordhoek E, Feng S, Zinner M, Banks PA. Relationship of necrosis to organ failure in
severe acute pancreatitis. Gastroenterology 1997; 113: 899-903 24 Beger HG, Bittner R, Block S, Büchler M. Bacterial
contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology 1986; 91: 433-438
25 Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute
pancreatitis. World J Surg 1997; 21: 130-135
26 Bassi C, Falconi M, Girelli R, Nifosi F, Elio A, Martini N, Pederzoli P. Microbiological find-ings in severe pancreatitis.
Surg Res Commun 1989; 5: 1-4
27 Meyers MA, Feldberg MA, Oliphant M. Grey Turner's sign and Cullen's sign in acute pancreatitis. Gastrointest Radiol
1989; 14: 31-37
28 Matull WR, Pereira SP, O'Donohue JW. Biochemical
markers of acute pancreatitis. J Clin Pathol 2006; 59: 340-344 29 Neoptolemos JP, Kemppainen EA, Mayer JM, Fitzpatrick
JM, Raraty MG, Slavin J, Beger HG, Hietaranta AJ, Puolakkainen PA. Early prediction of severity in acute
pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet 2000; 355: 1955-1960
30 Lempinen M, Stenman UH, Halttunen J, Puolakkainen P, Haapiainen R, Kemppainen E. Early sequential changes in
serum markers of acute pancreatitis induced by endoscopic retrograde cholangiopancreatography. Pancreatology 2005; 5: 157-164
31 Kylänpää-Bäck ML, Kemppainen E, Puolakkainen P, Hedström J, Haapiainen R, Korvuo A, Stenman UH.
Comparison of urine trypsinogen-2 test strip with serum lipase in the diagnosis of acute pancreatitis.
Hepatogastroenterology 2002; 49: 1130-1134
32 Lempinen M, Stenman UH, Finne P, Puolakkainen P, Haapiainen R, Kemppainen E. Trypsinogen-2 and
trypsinogen activation peptide (TAP) in urine of patients with acute pancreatitis. J Surg Res 2003; 111: 267-273 33 Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl
J Med 2006; 354: 2142-2150
34 Diehl AK, Holleman DR Jr, Chapman JB, Schwesinger WH, Kurtin WE. Gallstone size and risk of pancreatitis. Arch Intern Med 1997; 157: 1674-1678
35 Dholakia K, Pitchumoni CS, Agarwal N. How often are
liver function tests normal in acute biliary pancreatitis? J
Clin Gastroenterol 2004; 38: 81-83
36 Lucrezio L, Bassi M, Migliori M, Bastagli L, Gullo L.
Alcoholic pancreatitis: new pathogenetic insights. Minerva Med 2008; 99: 391-398
37 Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, Lande JD, Pheley AM. Complications of endoscopic
biliary sphincterotomy. N Engl J Med 1996; 335: 909-918 38 Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, Huang Q,
Zhang X, He LP, Sun WS, Zhao Q, Shi RH, Tian ZB, Li YQ, Li W, Zhi FC. Risk factors for ERCP-related complications: a
prospective multicenter study. Am J Gastroenterol 2009; 104: 31-40
39 Cappell MS. Acute pancreatitis: etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am
2008; 92: 889-923, ix-x
40 Balani AR, Grendell JH. Drug-induced pancreatitis : incidence, management and prevention. Drug Saf 2008; 31: 823-837
41 Parenti DM, Steinberg W, Kang P. Infectious causes of acute
pancreatitis. Pancreas 1996; 13: 356-371
42 Teich N, Mössner J. Hereditary chronic pancreatitis. Best Pract Res Clin Gastroenterol 2008; 22: 115-130
43 Schneider A, Barmada MM, Slivka A, Martin JA, Whitcomb DC. Clinical characterization of patients with idiopathic chronic pancreatitis and SPINK1 Mutations. Scand J Gastroenterol 2004; 39: 903-904
44 Talley NJ. Functional gastrointestinal disorders in 2007 and
Rome III: something new, something borrowed, something
objective. Rev Gastroenterol Disord 2007; 7: 97-105
45 Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med 2004; 351: 1218-1226
46 Larvin M, McMahon MJ. APACHE-II score for assessment
and monitoring of acute pancreatitis. Lancet 1989; 2: 201-205 47 Dervenis C, Johnson CD, Bassi C, Bradley E, Imrie CW,
McMahon MJ, Modlin I. Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini
consensus conference. Int J Pancreatol 1999; 25: 195-210 48 Uhl W, Büchler M, Malfertheiner P, Martini M, Beger HG.
PMN-elastase in comparison with CRP, antiproteases, and
LDH as indicators of necrosis in human acute pancreatitis.
Pancreas 1991; 6: 253-259
49 Al-Bahrani AZ, Ammori BJ. Clinical laboratory assessment
of acute pancreatitis. Clin Chim Acta 2005; 362: 26-48 50 Rau BM, Kemppainen EA, Gumbs AA, Büchler MW,
Wegscheider K, Bassi C, Puolakkainen PA, Beger HG. Early
assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg 2007;
245: 745-754
51 Rau BM. Predicting severity of acute pancreatitis. Curr Gastroenterol Rep 2007; 9: 107-115
52 Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer
FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139: 69-81 53 Imrie CW, Benjamin IS, Ferguson JC, McKay AJ, Mackenzie
I, O'Neill J, Blumgart LH. A single-centre double-blind trial
of Trasylol therapy in primary acute pancreatitis. Br J Surg
1978; 65: 337-341
54 Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter
DC. Prognostic factors in acute pancreatitis. Gut 1984; 25: 1340-1346
55 Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818-829
56 Wilson C, Heath DI, Imrie CW. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II,
clinical assessment and multiple factor scoring systems. Br J Surg 1990; 77: 1260-1264
factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology 2006; 6: 206-209 58 Johnson CD, Toh SK, Campbell MJ. Combination of
APACHE-II score and an obesity score (APACHE-O) for the
prediction of severe acute pancreatitis. Pancreatology 2004; 4: 1-6
59 Papachristou GI, Papachristou DJ, Avula H, Slivka A,
Whitcomb DC. Obesity increases the severity of acute
pancreatitis: performance of APACHE-O score and
correlation with the inflammatory response. Pancreatology
2006; 6: 279-285
60 Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction
and death in acute pancreatitis. Br J Surg 2006; 93: 738-744 61 Vincent JL, de Mendonça A, Cantraine F, Moreno R,
Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S.
Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a
multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care
Medicine. Crit Care Med 1998; 26: 1793-800
62 Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable
descriptor of a complex clinical outcome. Crit Care Med
1995; 23: 1638-1652
63 Peres Bota D, Melot C, Lopes Ferreira F, Nguyen Ba V, Vincent JL. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA)
score in outcome prediction. Intensive Care Med 2002; 28: 1619-1624
64 Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223: 603-613 65 Ranson JH, Balthazar E, Caccavale R, Cooper M. Computed
tomography and the prediction of pancreatic abscess in acute pancreatitis. Ann Surg 1985; 201: 656-665
66 Simchuk EJ, Traverso LW, Nukui Y, Kozarek RA.
Computed tomography severity index is a predictor of outcomes for severe pancreatitis. Am J Surg 2000; 179: 352-355
67 Werner J, Uhl W, Hartwig W, Hackert T, Müller C, Strobel O, Büchler MW. Modern phase-specific management of acute
pancreatitis. Dig Dis 2003; 21: 38-45
68 Kemppainen E, Sainio V, Haapiainen R, Kivisaari L, Kivilaakso E, Puolakkainen P. Early localization of necrosis
by contrast-enhanced computed tomography can predict outcome in severe acute pancreatitis. Br J Surg 1996; 83: 924-929
69 Uhl W, Roggo A, Kirschstein T, Anghelacopoulos SE, Gloor B, Müller CA, Malfertheiner P, Büchler MW. Influence of
contrast-enhanced computed tomography on course and outcome in patients with acute pancreatitis. Pancreas 2002;
24: 191-197
70 Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas
TN. Pancreatitis complicated by gland necrosis: evolution of
findings on contrast-enhanced CT. J Comput Assist Tomogr
1999; 23: 898-905
71 Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis.
Pancreas 2000; 20: 367-372
72 Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid
resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology 2002; 2: 104-107
73 Thompson DR. Narcotic analgesic effects on the sphincter of Oddi: a review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol 2001; 96: 1266-1272 74 Andriulli A, Leandro G, Clemente R, Festa V, Caruso
N, Annese V, Lezzi G, Lichino E, Bruno F, Perri F. Meta-analysis of somatostatin, octreotide and gabexate mesilate
in the therapy of acute pancreatitis. Aliment Pharmacol Ther
1998; 12: 237-245
75 Messori A, Rampazzo R, Scroccaro G, Olivato R, Bassi C,
Falconi M, Pederzoli P, Martini N. Effectiveness of gabexate
mesilate in acute pancreatitis. A metaanalysis. Dig Dis Sci
1995; 40: 734-738
76 Hughes CB, Grewal HP, Gaber LW, Kotb M, El-din AB, Mann L, Gaber AO. Anti-TNFalpha therapy improves survival and ameliorates the pathophysiologic sequelae in
acute pancreatitis in the rat. Am J Surg 1996; 171: 274-280 77 Hughes CB, Gaber LW, Mohey el-Din AB, Grewal HP, Kotb
M, Mann L, Gaber AO. Inhibition of TNF alpha improves
survival in an experimental model of acute pancreatitis. Am Surg 1996; 62: 8-13
78 Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role
of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock 2007; 28: 130-140 79 Hughes CB, el-Din AB, Kotb M, Gaber LW, Gaber AO.
Calcium channel blockade inhibits release of TNF alpha and improves survival in a rat model of acute pancreatitis.
Pancreas 1996; 13: 22-28
80 Norman J, Franz M, Messina J, Riker A, Fabri PJ, Rosemurgy AS, Gower WR Jr. Interleukin-1 receptor
antagonist decreases severity of experimental acute pancreatitis. Surgery 1995; 117: 648-655
81 Norman JG, Franz MG, Fink GS, Messina J, Fabri PJ, Gower WR, Carey LC. Decreased mortality of severe acute
pancreatitis after proximal cytokine blockade. Ann Surg
1995; 221: 625-631; discussion 631-634
82 Callicutt CS, Sabek O, Fukatsu K, Lundberg AH, Gaber L, Wilcox H, Kotb M, Gaber AO. Diminished lung injury with vascular adhesion molecule-1 blockade in choline-deficient
ethionine diet-induced pancreatitis. Surgery 2003; 133: 186-196
83 Lundberg AH, Fukatsu K, Gaber L, Callicutt S, Kotb M, Wilcox H, Kudsk K, Gaber AO. Blocking pulmonary
ICAM-1 expression ameliorates lung injury in established diet-induced pancreatitis. Ann Surg 2001; 233: 213-220 84 Vonlaufen A, Aurrand-Lions M, Pastor CM, Lamagna C,
Hadengue A, Imhof BA, Frossard JL. The role of junctional
adhesion molecule C (JAM-C) in acute pancreatitis. J Pathol
2006; 209: 540-548
85 Chen XL, Ciren SZ, Zhang H, Duan LG, Wesley AJ. Effect
of 5-FU on modulation of disarrangement of immune-associated cytokines in experimental acute pancreatitis.
World J Gastroenterol 2009; 15: 2032-2037
86 Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International
Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993; 128: 586-590
87 Stanten R, Frey CF. Comprehensive management of acute necrotizing pancreatitis and pancreatic abscess. Arch Surg
1990; 125: 1269-1274; discussion 1274-1275
88 Uhl W, Warshaw A, Imrie C, Bassi C, McKay CJ, Lankisch PG, Carter R, Di Magno E, Banks PA, Whitcomb DC, Dervenis C, Ulrich CD, Satake K, Ghaneh P, Hartwig W, Werner J, McEntee G, Neoptolemos JP, Büchler MW.
IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology 2002; 2: 565-573
89 Juvonen PO, Alhava EM, Takala JA. Gut permeability in
patients with acute pancreatitis. Scand J Gastroenterol 2000;
35: 1314-1318
90 Petrov MS, van Santvoort HC, Besselink MG, van der Heijden GJ, Windsor JA, Gooszen HG. Enteral nutrition
and the risk of mortality and infectious complications in patients with severe acute pancreatitis: a meta-analysis of
randomized trials. Arch Surg 2008; 143: 1111-1117
91 Eatock FC, Chong P, Menezes N, Murray L, McKay CJ, Carter CR, Imrie CW. A randomized study of early
nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am J Gastroenterol 2005; 100: 432-439