Periodontology 2000, Val. 2, 1993, 83-97
Printed in Denrnark . All rights reserved
C o p y r i g h t 0 Micnksgnard 1993
PERIODONTOLOGY 2000 ISSN 0906-6713
The role of local factors
in
the
etiology
of
periodontal diseases
KENNETH
S. KORNMAN
&
HARALD
LOE
Early concepts of periodontal disease were derived primarily from histopathological observations. Prominent pathologists interpreted the histopath- ology in various ways and produced somewhat diver- gent theories on the nature and causes of peri- odontitis (see Loe in this volume). Some scientists contended that periodontitis was the result
of
trauma from occlusion that produced excessive forces on the connective tissue and bone. Others believed that the disease had a systemic origin and was closely linked with systemic diseases. Some argued that certain forms of periodontitis were degenerative in nature and were therefore similar to other degenerative pro- cesses in the body. And for thousands of years, Egyp-tian, Hebrew and Chinese writings had spoken of in- dividuals who were “long of tooth” as a reference to individuals of old age. Some therefore argued that periodontitis was a natural consequence of aging. The accumulations of hard and soft material, includ- ing microorganisms, on tooth surfaces had been as- sociated with periodontal disease for many years and, beginning in the late 1800s, various peri- odontologists and microbiologists contended that parasites, protozoa, streptococci, spirochetes and certain black-pigmented anaerobes were responsible for periodontal disease. The presence of these vari- ous theories and their very vocal proponents resulted in varying concepts of therapy and very unpredict- able treatment outcomes. If a patient with peri- odontitis presented for treatment to one of the pro- ponents of trauma from occlusion, the patient might receive an occlusal adjustment and new dental crowns and bridges. Since some of these therapists would secondarily clean the teeth and others would not, the outcomes of this particular therapy were in- consistent.
During the first half of this century, with limited scientific evidence, multiple local factors were intro- duced as possible causes of periodontal pathology. There were really no means available at the time to de-
velop a clear understanding of the importance of any
specific factor or the relative influence of multiple fac- tors. The period that followed, beginning in the late 1950s and early 1960s, used the scientific method and hypothesis testing to clarify the dominance of primary correlations with disease, that is, plaque and age, which led to the demonstration that bacterial ac- cumulation is essential to disease initiation.
Although sophisticated experimental methods were rapidly applied to studies of periodontal dis- eases, experimental design and data analysis tech- niques were limited in their ability to evaluate the interaction of multiple factors. Such techniques for the study of the relative importance of multiple fac- tors in chronic diseases have emerged only in recent years and are still under development. These limi- tations, as well as the normal dialectic thought pro- cess, have produced somewhat different concepts of the role of local factors in periodontal disease at dif- ferent times.
Kornman & Liie
of the secondary factors. The net result was that, ex- cept for pregnancy and a few other systemic con- ditions, the influence of secondary factors may have been greatly discounted for many years.
As new information emerged in the 1970s and 1980s
on the potential role of certain bacteria and host re- sponses in the etiology of periodontal diseases, the story again became complicated. Much of the diffi- culty in attempting to determine the role of various factors may be due to the persistence of traditional concepts of disease etiology and data analysis. Some of the complicating factors in this interpretation in- volved the fact that different forms of periodontal dis- ease may have different interactions between the fac- tors involved in the disease. For example, in localized juvenile periodontitis, increasing supragingival plaque and age have a much weaker association with disease than traditionally found in adult periodontitis. Such observations are obvious for juvenile forms of periodontitis but have not been well defined and may be much more obtuse for variations of the disease in adults. In addition, the interaction between multiple factors may be nonlinear and complex. For example, the influence of smoking on disease may be of a differ- ent magnitude in some individuals than in others. In recent years, studies involving sophisticated ap- proaches to multivariate data analysis (28) and the de- termination of odds ratios have started to consider the influence of various combinations of multiple vari- ables on disease outcomes.
It therefore seems appropriate to reassess the role of local factors in the etiology of periodontal disease in light of the new knowledge and concepts of disease etiology as well as insights gained from new ap- proaches to data analysis. Unfortunately, limited data are currently available with these perspectives. The following discussion therefore represents the judgment of the authors based on the data that exist and is necessarily speculative. Many of the concepts remain to be proven with prospective studies. In many respects, the current concepts of the role of secondary factors in periodontitis are merely a re- finement of the concepts that emerged in the mid-
1960s, based on the increased understanding of the mechanisms by which secondary factors may influ- ence the disease process. The concepts discussed here emphasize the primary and essential role of bac- terial accumulation in initiating periodontal disease and attempt to clarify the specific interaction of sec- ondary factors in this process (22). In the future, new experimental designs and analytical techniques will further clarify the relative strengths of these various influences in individual patients.
Although it is assumed that local factors may func- tion differently in different forms of periodontal dis- ease, the discussion here focuses entirely on gingi- vitis and chronic adult periodontitis, since most of the available data are in this area. The relationship between various factors in adult periodontitis may be very different from that found in other forms of the disease.
In the past, the differentiation between local fac- tors and systemic factors may have seemed relatively clear. However, today it is recognized that many sys- temic factors act not only at the systemic level but may have differential effects at the local periodontal level. For example, steroid hormones produce a var- iety of systemic effects, including vascular and con- nective tissue changes and influences on the in- flammatory and immune systems. However, due to the density of steroid receptors in the gingival tissues
(137, 145) and the direct influence of steroid hor- mones on the microbial ecology in the gingival sulcus (641, hormones also exert a differential effect on the gingiva, which may be in addition to or different from its systemic influence. Similarly, the presence of cer- tain bacteria in the subgingival area may induce not only a systemic immune response but a somewhat different localized immune response in the gingival area (30, 46, 67). It therefore seems that the lines between local and systemic factors have become blurred in many instances. For the purposes of this discussion, we attempt to focus on factors in which the dominant effect is primarily local. Thus, local fac- tors refers to anything that influences the periodontal health status and is reflected and may be measured locally but has no overt systemic influence.
This discussion also assumes that chronic adult periodontitis refers to people over the age of 35-40 years who have clinical evidence of plaque and calcu- lus and of loss of attachment and supporting bone. This classification also assumes certain biological boundaries, including the absence of overt systemic disease, the absence of overt problems with host de- fense mechanisms and the assumption that, in an
intact host, there are redundant mechanisms for pro- tecting the host from bacterial insults.
Other characteristics of chronic adult periodontitis are assumed as part of the definition used here. These include the concept that essentially everyone who accumulates plaque will develop gingivitis (79)
and that most of these individuals will ultimately pro- gress to chronic adult periodontitis (10, 72, 82, 83).
The role of local factors
chronic adult periodontitis group. There is substan- tial evidence in chronic adult periodontitis that plaque accumulates and matures ecologically in a predictable pattern (76, 103, 139, 141, 142). Both gin- givitis and periodontitis have been strongly corre- lated with the maturation of plaque and the specific bacterial components characteristic of later stages of maturation. The role of local factors in the etiology of periodontitis is discussed in light of these boundary conditions. However, recent studies involving twins
(98) indicate that genetic factors play a prominent
role in both gingivitis and periodontitis, and these findings apparently apply to chronic adult peri- odontitis. Although this is critical information, the role of the genetics of the host is not directly dis- cussed here (see Genco & Loe in this volume).
Classification of local
etiological factors
Given the strength of evidence supporting the re- lationship between plaque accumulation and peri- odontitis,
a
simple coherent concept developed that described periodontitis as the outcome of persistent inflammation resulting from plaque accumulation. It was recognized, however, that some individuals with host differences, such as localized juvenile peri- odontitis, exhibited a different etiology. In addition, even for chronic adult periodontitis patients, in which no overt host problem exists, lossof
attach-ment and disease progression could not be explained solely by the presence of accumulated plaque (51,
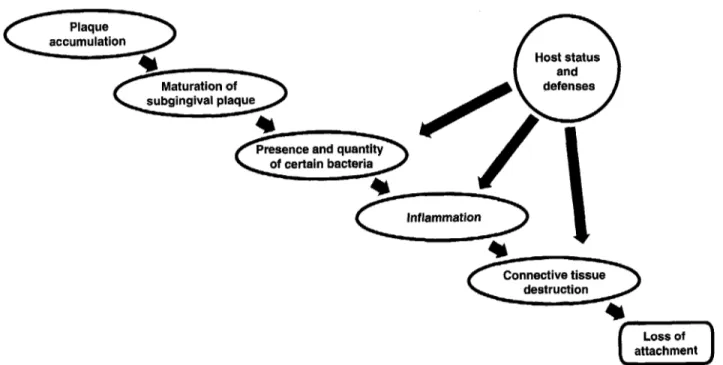
74). As a result, recent efforts have attempted to cor- relate the presence of certain bacteria with disease initiation and progression. Although extensive en- ergy has been devoted to this effort in chronic adult periodontitis, clear correlations between specific bacteria and disease initiation and progression are still somewhat elusive. Fig. 1 depicts one view of the current situation. The arrows do not represent causality but show where one factor influences an- other factor. Fig. 1 may be explained as follows.
As plaque accumulates, gingival inflammation is initiated and the plaque begins a maturation process that is both predictable and repeatable. This matu- ration of plaque leads to ecological changes that re- sult in the detection and increase of certain groups of bacteria that have pathogenic potential. These bacteria augment the inflammatory process. Con- nective tissue destruction results from nonspecific inflammatory mechanisms as well as more specific inflammatory mechanisms related to certain bac- teria. The nature and extent of the connective tissue destruction is also influenced by host characteristics. This map describes the initiating factors respon- sible for the loss of attachment in chronic adult peri- odontitis. In recent years all other factors have been relegated to the role of secondary or modifying in- fluences on the disease process. Modifying factors influence some aspect of the primary initiating fac- tors. Fig. 2 lists some of these modifying factors under
n
Host statusInflammation
destruction
Loss of
0
attachmentKornman & Lae
Primary disease
influences
accumulation destruction
Modifying Influences Modifying Influences Modifying Influences
Oral hygiene Maturatlon of marginal plaque Genetlc Influence
Tooth malposition Host defenses lntlammatlon
Tooth anatomy Pocket depth Particular bacterla
Restoratlons Restorations Smoking
Gingival contours Subgingival environment Calculus
Calculus
Smoking
Fig. 2. Local factors in chronic adult periodontitis
the primary manner in which they influence the dis- ease process. For example, oral hygiene, tooth mal- position, tooth anatomy, gingival contours and over- hanging restorations most likely alter plaque accumulation. The presence and quantity of certain bacteria, some of which have periodontal pathogenic potential, are primarily influenced by the plaque maturation but have also been shown to be influ- enced by pocket depth (86, 1021, calculus (1101, over- hanging restorations (68) and the nutritional and en- vironmental characteristics of the subgingival area
(84,85). There is also some preliminary evidence that smoking may alter the bacterial ecology (66). Con- nective tissue destruction may be influenced by the nature of the inflammation process, the presence and quantity of certain bacteria, the nature of an in- dividual’s anabolic and catabolic balance and local modifymg factors (Fig. 2 ) .
The nature of the interactions in Fig. 2 demonstrate that evaluation of the role of local factors in the etiol- ogy of disease is complex and cannot be easily as- sessed, even with the most sophisticated statistical models. For example, as seen in the single-variable studies of the 1960s and 1970s the role of secondary factors may be easily overwhelmed by the dominance of primary factors. This sometimes resulted in the conclusion that the secondary factors were not sig- nificantly associated with disease. Recent approaches to the analysis of epidemiological data may provide a more realistic view of potential secondary influences. For example, statistical isolation and combination of multiple factors allow one to conclude that, although smoking does not initiate periodontitis, it significantly increases the risk of disease (9).
Thus, it is now easy to understand how secondary local factors have either been discounted as having
nothing to do with periodontitis or have been confus- ing due to inconsistent correlations with disease. The proper assessment of their role must involve both multifactorial experimental designs and multifacto- rial analysis. Few studies meet these criteria, and therefore much of the evaluation of the role of sec- ondary factors must involve extrapolation of the existing studies.
Although periodontal inflammation is initiated by plaque accumulation, the severity of inflammation and the extent of periodontal tissue destruction that results is determined by the nature of the host re- sponse of the specific individual patient. In recent years, epidemiological studies from various popula- tions have well documented that the prevalence of diseased sites varies greatly among individuals and that variation is not explainable solely by the amount of dental plaque (51, 73, 83, 98, 99).
Given the above perspective, it is now reasonable to reconsider the potential role of individual local factors in the etiology of periodontal disease.
Local factors that primarily
influence plaque accumulation
The role of local factors
shown in Fig. 2. The factors that influence the amount of dental plaque that accumulates supragingivally are oral hygiene, tooth malposition, tooth anatomy, over- hanging restorations and gingival contours.
Oral hygiene
Good oral hygiene, in particular good toothbrushing, has long been associated with better periodontal health than poor oral hygiene. However, before the critical role of plaque accumulation had been dem- onstrated, some investigators contended that oral hygiene practices may prevent disease by stimulating the gingiva (39). Numerous studies from the late 1950s to the early 1980s demonstrated quite convinc- ingly that oral hygiene influences disease primarily by eliminating or reducing plaque accumulation (6, 79, 88, 138, 141).
Tooth malposition
Various parameters of malocclusion or tooth malpo- sition have been correlated with periodontitis (19,35, 36). Earlier concepts suggested that this relationship may actually involve traumatic occlusion (401, but it is now generally accepted that the primary effect of malposition is the effect on plaque accumulation due to more difficult cleaning around malpositioned teeth (20, 60, 128).
Tooth anatomy
Several factors related to tooth anatomy, including root formation, such as enamel projections (94), lin- gual grooves (31, 701, root depressions (45) and fur- cations (61, 62, 113) and others (571, have been his- torically associated with periodontitis. Enamel projections, lingual grooves and furcations probably primarily influence the accumulation of plaque, since the anatomy provides a surface configuration with little access for cleaning (124). This protected area allows plaque to accumulate, mature, calcify partially or completely and retain toxins; the patient has limited access to cleaning this area and great ef- fort is required to clean it professionally. Although such anatomic areas may also have altered fiber re- lationships, proper cleaning of these areas or alter- ation of the area to allow proper cleaning reduces the risk of disease (90). Such observations suggest that the primary influence of these anatomic factors is on plaque accumulation.
Tooth anatomic factors such as plunging cusps and open contacts that potentially allow food impac-
tion have also been associated with periodontitis (69, 109). It is unclear whether such associations are the result of disease or whether they actually contribute to the disease. If the latter is true, the mechanism of such an association is unclear.
Overhanging restorations
Almost 40 years have passed since Waerhaug (147)
provided the first scientific evidence that dental res- torations placed in a subgingival location were detri- mental to periodontal health. Since that time much clinical (4, 15, 91, 1271, histological (149) and bac- teriological work (68) has confirmed the close associ- ation between periodontal disease and the subgingi- Val margins of fillings, crowns and inlays. Damage to the periodontal tissue might occur during the prep- aration and fabrication of the restorations; the ma- terials used might contain components that irritate tissue; and the physical or chemical properties of the restorations may cause retention of bacterial plaque in the long term.
Preparation with any rotating instrument below the gingival margin represents a trauma of varying degree to the crevicular epithelium and the subepith- elial connective tissue. Similar acute damage is pro- duced by the use of retraction materials when im- pressions are made or when temporary restorations are worn. Such lesions are, however, considered to be reversible, and if the local environmental con- ditions are favorable, a new epithelium proliferates to cover the exposed connective tissue wound. Com- plete healing normally occurs within 8-14 days (80).
Studies on the gingival tissue response to dental materials commonly used in restorative dentistry (cast gold, gold foil, porcelain and heat-cured acrylics) indicate that they are inert. Others may cause slight acute injury, either through the leakage of specific components from the material (such as monomers) or the release of corrosion products (43). Allergic reac- tions of the oral mucosa (including the gingiva) to dental materials have been seen in a few patients, but most materials in use today are well tolerated by the vast majority of the patients and therefore cannot ex- plain the destruction of the periodontal tissues ad- jacent to fillings, inlays and crowns.
Kornman & Lije
these restorations exert any direct injury to the local tissues but rather that the rough surfaces invite the colonization and retention of periodontal pathogens, and the detrimental effect of this is seen in the tissue response. Studies have shown that the microflora ad- jacent to subgingival overhangs are very similar to the bacterial composition of plaque from patholog- ical pockets.
There are some indications that, in patients with good oral hygiene, gingival reactions to subgingival fillings and ill-fitting margins are milder than in pa- tients who do not practice active oral self-care on a regular basis (4).
Thus, overhanging restorations appear to in- directly influence the disease in two primary ways. Areas with overhangs are difficult to clean and have repeatedly been associated with more plaque than unrestored or clinically acceptable restorations (14,
38). Overhangs may also influence plaque matu- ration more directly by changing the environment to promote the accumulation of certain types of bac- teria (76). This has been demonstrated by studies (68) in which overhanging restorations, but not clinically acceptable restorations, allowed an increase in pig- mented bacteria such as Prevotella intermedia and
Porplzyromorzas gingivalis that are routinely detected in later stages of plaque maturation.
Gingival contours
Although early toothbrushing techniques such as the press-and-roll technique were complicated by the presence of bulky gingival contours, there is no evi- dence that more current techniques, if properly ap- plied, such as the modified Bass technique, are al-
tered in any say by such contours. The concept that gingival contours interfered with plaque removal has been used historically as one of the rationales for re- contouring both bone and gingiva (42, 108, 122). It should be noted that, for current cleaning techniques, there is no evidence that gingival contours interfere with cleaning and therefore result in more plaque ac-
cumulation. On the other hand, once disease has been established, more root surface area exists to be cleaned by either the professional or the patient.
odontitis, the actual correlation between supragin- gival plaque levels and longitudinal disease progression has been found to be rather weak (73,74). This is to be expected, since supragingival plaque is measured but the pathogenic bacteria are localized in the subgingival plaque, which is not clinically measur- able. In addition (Fig. 11, plaque accumulation does not directly result in loss of attachment but influences destruction through its effects on the presence and quantity of pathogenic bacteria, which is modulated by the nature of the host response. Therefore, simple correlations between supragingival plaque levels and loss of attachment should not be expected to be strong because of the number and complexity of intervening influences. If a population group with minimal plaque is compared with one with substantial plaque, the population that has more plaque will have substan- tially more disease (120, 121). These studies reaffirm the essentiality of plaque but do not address the issue of the nature of the relationship between the presence of plaque and disease initiation or progression.
The role of specific types of
bacteria in adult periodontitis
Beginning in 1975 with the identification of certain bacteria associated with localized juvenile peri- odontitis, there has been a focus on the role of se- lected bacteria in periodontal disease. Although the etiological role of Actinobacillus actinomycetemcomi- tans in localized juvenile periodontitis has been well documented over the years (65, 107, 129, 151), the efforts to associate certain bacteria with the initiation of adult periodontitis have been both rewarding and frustrating.
Rewards and frustration
Substantial evidence exists that a limited number of bacteria (Table 1) are associated with periodontal diseases in adults on a cross-sectional basis (133, 135). In addition, if detectable levels of some of these
Table 1. Bacteria most frequently associated with
ueriodontal disease in adults Significance of plaque accumulation
Many of the local factors that have been previously as- sociated with periodontal disease appear to influence the disease process by affecting supragingival plaque accumulation and its removal. Although supragin- gival plaque is essential to the initiation of adult peri-
Actinobacilliis actinomycetemcomitans Porphyronionas gingivalis
Prevotelln intermedia Bacteroides forsytlzus Campylobacter rectirs Treponenia species
The role of local factors
species are present at an individual site, it is more likely to show disease progression (58, 150). In ad- dition, following therapy, sites in which selected bac- teria are eliminated respond better than sites in which these types of bacteria remain detectable (23, 37, 130, 131, 134).
Despite this very strong evidence that certain bac- teria are somehow involved in the disease process of adult periodontitis, substantial questions remain and have produced frustration both for researchers and practitioners attempting to use this information. For example, attempts to associate specific bacteria with disease progression have produced conflicting re- sults (29, 44, 52-55). It is relatively easy to demon- strate that the species present in periodontal pockets differ from those in healthy gingival sulci, but since the ecology is dramatically different in the two situ- ations it is difficult to draw conclusions about causal- ity just because bacterial differences exist. The more stringent challenge would be to follow gingivitis sites with and without specific putative pathogens to de- termine whether there is a difference in progression. In humans (150), sites with P. intermedia, P. gingi- valis or A. actinomycetemcomitans show significant clinical progression in about 20% of the sites over 12 months, whereas none of the sites without detectable levels of those microorganisms exhibited pro- gression. In animals, the implantation of P. gingivalis
into a pre-existing gingivitis microbiota results in rapid disease progression (58). However, when one looks at the actual correlations between disease pro- gression and certain bacteria (54, 55, 150) on a pro- spective basis, the correlations are rather weak. In addition, P. intermedia, which is one of the micro- organisms most implicated in disease in adults (1341, is frequently present without producing any ap- parent problem (150).
Potential explanations for the role of specific bacteria
It is of interest that individual periodontal sites are colonized by large numbers of bacteria, many of which have pathogenic potential, and yet progressive periodontal destruction occurs either rarely or at a rate at which clinical detection is infrequent. The functional relationship between individual bacteria or the composite microbiota and then destruction of the periodontium is undoubtedly complex, because the bacterial challenge is translated differently by each individual host. However, assessing the bac- terial challenge by growing and biochemically identi- fying microbial species may produce too crude a level
of specificity to properly characterize the bacterial challenge. In recent years, genetic analysis has shown both A. actinomycetemcomitans (56, 151) and P. gin- givalis (87) to be very heterogeneous.
The factors that may influence both the quality and quantity of the bacterial challenge in periodontal disease were reviewed recently (134). It is important to first recognize that, with few exceptions, previous associations between bacterial species and peri- odontal disease have been based on species-level identification of the microorganisms. Experience in other diseases has clearly indicated that many clonal types may be contained within a single species. Most importantly, within a given species only a few clonal types appear to be pathogenic (18, 33, 105). For ex- ample, it has been known for many years that micro- organisms currently classified as P. intermedia ex- hibit great genetic variability (24, 411, yet the relationship of the different genotypes to periodontal disease is essentially unknown. To understand the true correlation between certain bacteria and dis- ease, it is essential to first determine the relative viru- lence potential of the various clonal types within a species suspected of being pathogenic. Several attempts have been made in recent years to evaluate the pathogenic potential of various strains within P.
gingivalis (50, 92, 97, 106, 126, 132, 144). A diversity among A. actinomycetemcomitans strains relative to genetics, distribution in the population and leuko- toxic activity has been observed for several years (56, 151). Although studies in animal model systems rep- resent important first steps, the relationships be- tween different clonal types, animal virulence models and the actual induction of periodontal disease re- main essentially unexplored.
Kornrnan & Lije
levels, but species that counter some of the effects of the pathogens may be altered, resulting in a change in the net bacterial challenge. Finally, it has been well documented that specific regulator genes may switch on an entire response pattern within a microorganism to allow it to cope with the specific environment (95, 101). Environmental changes that appear to be subtle at the host level may be sufficiently critical at the level of the microbiota to switch on genes that increase or decrease an entire set of virulence factors (59). Recent studies in periodontal microorganisms have observed similar phenomena (8, 17,961.
With these concepts in mind, it is very reasonable to expect to detect a suspected pathogen in many sites, but only a subset of these sites house the geno- types and environmental factors essential for viru- lence. Even if these stringent requirements are met at any given time, the host response at that particular time must be such that destruction occurs. This above scenario appears unusually stringent and may appear unnecessarily complicated because the con- tributing factors are described as independent vari- ables. However, the probability of achieving all of the factors necessary for disease may increase substan- tially if many of the bacterial requirements for dis- ease are controlled in common by an outside factor. For example, the normal maturation of the subgingi- Val plaque and the gingival response to this matu- ration process may provide much of the microbial ecology and physical and biochemical factors necess- ary for an optimal virulence state in an ecosystem. Under such a scenario, the number of independent variables that must be aligned to achieve virulence would drop dramatically and the probability of achieving a virulent state should increase substan- tially. Extensive studies in the coming years will un- doubtedly clarify both of these scenarios. For the mo- ment, it seems that the important conclusion is that simplistic associations between selected bacterial species, as currently identified, and the initiation and progression of periodontal disease are not impress- ively strong. This should not be taken to mean that specific bacteria are unimportant in the disease pro- cess, but rather that the previous views of the re- lationships were most likely overly simplistic.
Factors influencing the
presence and quantity of
specific bacteria
The presence and level of particular bacteria in the subgingival microbiota are a function of the bacteria
acquired at some point by the individual and the en- vironmental and host factors that determine the eco- logical balance within the microbiota. If supragin- gival plaque is allowed to accumulate undisturbed, the subgingival microbiota develops in a rather pre- dictable and consistent pattern (79, 141) that varies from site to site and individual to individual (103, 139). A variety of physical and chemical factors pro- vided by the host and other bacteria have been de- scribed that appear to be critical to certain interac- tions between the bacteria during this ecological development (84). Local factors that influence certain bacteria in the subgingival area and that may be under the control of the patient or the therapist in- clude probing depth, overhanging restorations and maturation of the plaque. The influence of over- hanging restorations and of plaque maturation have been discussed above.
Pocket depth
Although there has been some confusion in recent years about the likelihood of disease progression in sites of different pocket depth, several recent studies have confirmed the longstanding clinical impression that sites with a previous history of disease are more likely to show future progression than sites with no previous disease history (1, 27, 53-55, 73, 74, 114). In untreated periodontal pockets, the most obvious influence of pre-existing disease on future disease may be the presence of substantial bacterial loads within the pocket. Previous studies (85, 86) have demonstrated that different bacterial patterns and different ecological factors may be correlated with the pocket depth. Although many specific, and as yet undefined, factors may explain this relationship, it does appear that the microbiota in untreated deeper pockets shifts towards a more anaerobic population. Nevertheless, there is no evidence that these relation- ships exist following therapy that has cleaned out the subgingival plaque. In other words, the critical ques- tion seems to be whether or not deeper pockets re- colonize faster following subgingival cleaning. At present there is no evidence to suggest that pocket depth is a critical determinant of disease progression following therapy.
Calculus
The role of local factors
and severity of periodontal disease (48, 78, 88, 120, 121, 146).
Clinical surveys in many parts of the world showed that dental calculus was common, although the amount and location might vary (47, 77, 88, 93, 115, 118). In general, supragingival calculus was thought to be more prevalent than subgingival calculus, and the percentage of persons with either type increased with age (123).
The natural history of calculus formation in the absence of oral care
Prospective longitudinal studies of calculus forma- tion in tea workers in Sri Lanka, who never received dental or periodontal care and did not practice self- care during 45 years of life, have yielded new infor- mation on the natural history of calculus formation (3, 81). Under such circumstances, calculus forma- tion starts early. At the age of 14 years, all participants had supragingival calculus. The first teeth to show calculus were mandibular incisors and maxillary first molars. The time in the life of these teeth at which calculus first formed could not be ascertained, since this had already occurred in the youngest study sub- jects. However, based on sequential assessments of calculus formation on other tooth types, it is clear that, without deliberate interference, supragingival calculus formation starts very soon after the tooth has erupted.
When the subjects were 25 years of age, calculus was found on most teeth, and the differences be- tween first and second molars had disappeared. Maxillary incisors and bicuspids of both jaws might still be calculus-free, and even when the subjects were 45 years of age a few of these teeth were still without calculus, although the general calculus for- mation was massive.
Irrespective of age, the distribution of calculus was symmetrical by tooth type and surface. No difference in formation rates was seen between the mesial, dis- tal and lingual surfaces. However, the mean score for the buccal surfaces was consistently the lowest.
In this population the phase of supragingival cal- culus formation was brief, and subgingival calculus also started early, in most instances within 6 to 8 years of the eruption of the tooth. Subgingival calcu- lus was first found on the interproximal root surfaces as a subgingival continuation of an already existing supragingival deposit or as a separate and indepen- dent subgingival entity. By the age of 30 years and beyond, however, subgingival calculus was found on
virtually all root surfaces of all types of teeth without any special patterns.
Calculus formation in the presence of oral health care
The Norwegian men participating in a longitudinal investigation had received professional oral health care at regular schedules prior to and during the 20
years of the study. They all reported that they had practiced oral hygiene measures daily during most of their lives.
Of the 565 men between 16 and 34 years who par- ticipated in the first examination in 1969, only 7 indi- viduals were completely calculus-free, and in the 558 who had either supragingival or subgingival calculus or both, the mean Calculus Index was low and the distribution within the dentition was very limited.
About one third of the 16- to 17-year-olds ex- hibited the classical location of supragingival calcu- lus in mandibular incisors and maxillary first molars. However, supragingival calculus was 6 times more prevalent in mandibular incisors than in maxillary molars. Supragingival calculus was rarely seen in other types of teeth. In this population supragingival calculus did not increase with age. Rather, the indi- vidual Calculus Index scores tended to change from zero to positive or from positive to zero between examinations, most likely reflecting the regularity of visits to the dental office.
Subgingival calculus was rarely observed in adoles- cents who received optimal dental care. In 16- to 17- year-olds, about 3% of the mandibular incisors had subgingival calculus. This increased to involve ap- proximately 25% of these teeth as the men ap- proached 50 years of age.
The fact that subgingival calculus also first oc- curred on mandibular incisors and maxillary molars might suggest that the initial supragingival deposits had created the conditions for subgingival calculus formation. This is not to imply that supragingival cal- culus is a prerequisite for subgingival calculus forma- tion. On the contrary, as this and other studies (123) have shown, subgingival calculus regularly forms without being preceded by the supragingival variety. However, when it occurs, it is conceivable that the initial supragingival deposit may have created con- ditions for subsequent formation of subgingival cal- culus. At any rate, subgingival calculus was rare in this group. When the men were 25 years of age, 1-2 interproximal surfaces were involved; as the men ap- proached 50 years of age, 4-5 sites were affected.
Kornman & Liie
do not receive professional oral health care and who essentially practice no or little oral hygiene start forming supragingival calculus shortly after tooth eruption; if left untreated, it will continue to grow, seemingly governed only by time and available space. Under such circumstances, subgingival calcu- lus may be seen 6-8 years after eruption of individual teeth and increases in severity and extent with time. In contrast, the practice of good oral hygiene and frequent check-ups and professional care were as- sociated with very low levels of both supra- and sub- gingival calculus. However, the relative contribution of professional and personal care to this outcome is still not clear.
Recent cross-sectional studies have indicated that similarly low levels of calculus are experienced by the majority of populations in which oral hygiene is actively practiced and oral health care services are provided and utilized. Approximately 50% of adults of both sexes in the United States and Denmark had no calculus (63, 100). When calculus occurred, about one third of the available tooth surfaces were affect- ed. Studies of calculus in developing countries have reported prevalence rates ranging from moderate to extreme (5, 7, 25, 71, 136). N o populations or major group of individuals have yet been seen who, in the absence of active prevention or removal, go through life without calculus.
Mechanisms of action
Supragingival calculus is almost always associated with gingivitis or periodontitis (47, 1191, and subgin- gival calculus is invariably associated with loss of periodontal attachment and pathological pocket for- mation (89, 146). The longitudinal studies in Sri Lan- ka and Norway generally confirmed these relation- ships and demonstrated that, when calculus formation is allowed to occur without interruption, subgingival calculus is associated with higher rates of progression of the periodontal lesion. On the other hand, low levels of supragingival calculus are associ- ated with high levels of gingival health, and scattered, small amounts of subgingival calculus do not seem to influence significantly the progression of the peri- odontal lesion (2, 3, 82).
It was long maintained that calculus caused gingi- val inflammation by mechanical irritation and that the rough mineralized surfaces of calculus produced ulcerations in the gingival tissues. It was shown, first microscopically (146), that the mineralized part of both supragingival and subgingival calculus are not in contact with the periodontal tissues, and that cal-
culus is invariably covered by a soft, nonmineralized plaque that lies in immediate contact with the epi- thelial cells of the gingival sulcus. Subsequently, elec- tron microscopic pictures of these relationships re- vealed that these soft deposits consist largely of microorganisms (140).
It was also demonstrated that rough surfaces on teeth do not per se cause injury to the gingival epi- thelium (148) but primarily serve as retention sites for oral microorganisms. This was confirmed in a 2- year clinical trial of daily oral use of an antibacterial agent (chlorhexidine). Under these experimental conditions supragingival calculus formed, but due to the antibacterial action of chlorhexidine, its surface was not covered by live bacteria and the gingiva re- mained healthy (81). Others (75) have reported that, in monkeys treated with chlorhexidine, histological studies revealed a normal junctional epithelium at- tached to subgingival calculus.
Although calculus may not directly influence peri- odontitis, it would be reasonable to expect that sub- gingival calculus would influence the physical chemi- cal environment and therefore the microbial ecology of the subgingival region. Since calculus has the po- tential to concentrate both nutrients and toxins, (1 16) one might expect substantial influences on the bac- terial ecosystem. Although this may be the case, it is truly speculative.
Connective tissue destruction
The accumulation of a bacterial load and specific bacteria and associated gingival inflammation ap- pear to be essential for adult periodontitis, but the transition from gingivitis to periodontitis is unlikely to be a simple function of whether or not the in- flammation has extended into the supporting struc- tures (49). Inflammation is a composite term that in- cludes many redundant pathways that together produce clinical signs and symptoms (112). The pro- cess involved in destroying supporting tissues rather than just the changes involved in gingivitis is not known. Although it is well established that certain inflammatory pathways contribute to bone destruc- tion (104, 112), the key question is what factors shift the balance from a chronic gingivitis state to one of destructive periodontitis.
The role of local factors
ent with the observation that gingivitis may be stable for years without evidence of progression to peri- odontitis (21, 72) and that bacterial changes (58) and host changes, such as in some individuals with hu- man immunodeficiency virus infection, may result in progressive destruction.
Other factors (Fig. 2) are potential secondary in- fluences on the destructive process. For example, smoking (see Genco & Loe in this volume) is known
to alter the inflammatory process (16, 125, 143), but the influence of these effects on periodontal disease has not yet been defined. Studies (32) have shown that smokers have more calculus, deeper pockets and more bone loss but less clinical inflammation and supragingival plaque than nonsmokers. Even when adjusted for age and calculus, the smokers were found to have less inflammation and more bone loss than the nonsmokers. Multiple studies have demon- strated that smoking does not alter the clinical levels of plaque accumulation but results in less gingival inflammation than observed in nonsmokers (11, 13, 26, 32, 89). If one were to substantially reduce the bacterial challenge in both smokers and nonsmokers, it would undoubtedly be found that smoking by itself is not a primary factor in connective tissue destruc- tion. However, even in subjects with good oral hy- giene, smokers had significantly more bone loss than nonsmokers (12), and smoking alters the clinical re- sponse to therapy (117). These studies suggest that smoking may have a substantial modifying effect on the process of connective tissue destruction once it has been initiated.
In addition to the role of calculus in altering the bacterial ecosystem as discussed above, calculus may influence the connective tissue destructive process. One means is by concentrating bacterial toxins (116) in such a way that calculus actually increases the net bacterial challenge presented to the host, above and beyond the challenge expected from the plaque alone. In addition, by calcifying successive layers of plaque, calculus formation extends the bacterial front and may therefore be one mechanism for shift- ing the bacterial challenge and the zone of destruc- tion (1 11) more apically.
Conclusion
The following conclusions on the role of local factors in the initiation and progression of periodontal dis- ease appear to be appropriate at this time:
0 The definition of a local factor has become increas-
ingly complex as the understanding of host re- sponses in periodontal disease and in inflam- mation in general has increased.
0 It is now recognized that local factors other than
bacterial plaque may play important modifymg roles in specific individuals. Unfortunately, until recently, study designs and analytical techniques have limited the ability to assess the complex inter- actions necessary to evaluate the true role of some of these factors in periodontal disease and therapy. This process of understanding the role of modifiers of disease is complicated even more by the fact that, as multiple factors become involved, the in- fluence on disease outcome may involve a complex function that is not linear and may change at differ- ent stages of the disease process.
We therefore enter a relative new phase where we hope to clarify more specifically not only the role of bacterial plaque and certain pathogenic bacteria in the initiation and progression of periodontitis but also the role of systemic and local modifymg factors and their magnitude of influence in individual pa- tients.
Acknowledgements
We appreciate the assistance of Dr. Stephen Bass and Ms. Elaine Robertson in manuscript preparation.
References
1.
2.
3. 4.
5. 6. 7.
Albandar JM. A 6-year study on the pattern of periodontal disease progression. J Clin Periodontol 1990: 17: 467471. Anerud A, Loe H, Boysen H, Smith M. The natural history of periodontal disease in man. Changes in gingival health and oral hygiene before 40 years of age. J Periodont Res 1979: 14: 526-540.
Anerud A, Loe H, Boysen H. The natural history and clin- ical course of calculus formation in man. J Clin Peri- odontol 1991: 18: 160-170.
Arneberg D, Silness J, Nordbi H. Marginal and crevical extent of class 11 amalgam restorations related to peri- odontal conditions. A clinical and roentgenological study of overhang elimination. J Periodont Res 1980: 15:
Arthayukti P, Pariyakanok P, Triratana T. Calculus ac- cumulation on tooth surface in children. J Dent Assoc Thailand 1991: 41: 10-17.
Kornman & Loe
8. Barua PK. Dyer DW, Neiders ME. Effect of iron limitation on Bacteroides girzgiinlis. Oral Microbiol Immunol 1990: 9. Beck JD, Koch GG, Zambon JJ, Genco RJ, Tudor GE. Evaluation of oral bacteria as risk indicators for peri- odontitis in older adults. J Periodontol 1992: 63: 93-99. 10. Becker W, Berg L, Becker BE. Untreated periodontal dis-
ease. A longitudinal study. J Periodontol 1979: 50: 11. Bergstrom J , Preber H. The influence of cigarette smoking
on the development of experimental gingivitis. J Peri- odont Res 1986: 21: 668-676.
12. Bergstrom J, Eliasson S. Cigarette smoking and alveolar bone height in subjects njth a high standard of oral hy- giene. J Clin Periodontol 1987: 14: 466-469.
13. Bergstrom J. Oral hygiene compliance and gingivitis ex- pression in cigarette smokers. Scand J Dent Res 1990: 98: 497-503.
14. Bjorn AL, Bjorn H, Grkovic B. Marginal fit of restorations and its relation to periodontal bone level. Part 11. Crowns. Odontol Revy 1970: 21: 337-346.
15. Bjorn AI., Bjorn H. Grkovic B. Marginal fit of restorations and its relation to periodontal bone level. I. Metal fillings. Odontol Revy 1969: 20: 311-321.
16. Bosken CH, Hards J . Gatter K. Hogg JC. Characterization of the inflammatory reaction in the peripheral airways of cigarette smokers using immunocytochemistry. Am Rev Respir Dis 1992: 145: 911-917.
17. Bramanti TE, Holt SC. Iron-regulated outer membrane proteins in the periodontopathic bacterium, Brrcreroides girlgildis. Biochem Biophys Res Commun 1990: 166: 18. Brenner DJ, hlayer LW, Carlone GM et al. Biochemical.
genetic, and epidemiologic characterization of Hrrerno- pliilcts itifliierizae biogroup aegypriits (Hnernopliiliis negyprius) strains associated with Brazilian purpuric fe- ver. J Clin Microbiol 1988: 26: 1524-1534.
19. Buckley LA. The relationship between malocclusion and periodontal disease. J Periodontol 1972: 43: 413-417. 20. Buckley LA. The relationship between malocclusions,
gingival inflammation, plaque and calculus. J Periodontol 21. Buckley LA, Crowley >IJ. A longitudinal study of untreated periodontal disease. J Clin Periodontol 1984: 11: 523-530. 22. Caton JG, Quinones CR. Etiology of periodontal diseases.
Curr Opin Dent 1991: 1: 17-28.
23. Christersson LA, Zambon JJ, Genco RJ. Dental bacterial plaques. Nature and role in periodontal disease. J Clin Periodontol 1991: 18: 441-446.
24. D a h l h G, Wikstrom M, Renvert S, Gmiir R, Guggenheim B. Biochemical and serological characterization of Bricfer-
oides irirerrnediits strains isolated from the deep peri- odontal pocket. J Clin Microbiol 1990: 28: 2269-2274. 25. Dahllof G. Bjorkman S , Lindvall K. M o E, Modeer T. Oral
health in adolescents with immigrant background in Stockholm. Swed Dent J 1991: 15: 197-203.
26. Danielsen B, Manji F, Nagelkerke N. Fejerskov 0, Baelum V. Effect of cigarette smoking on the transition dynamics in experimental gingivitis. J Clin Periodontol 1990: 17: 27. Deas DE, Pasquali LA, Yuan CH, Kornman KS. The re- lationship between probing attachment loss and compu- terized radiographic analysis in monitoring progression of periodontitis. J Periodontol 1991: 62: 135-141. 5: 263-268.
234-244.
1146-1154.
1981: 52: 35-40.
159-161.
28. DeRouen TA, Mancl L, Hujoel P. Measurement of as-
sociations in periodontal diseases using statistical methods for dependent data. J Periodont Res 1991: 29. Dzink JL, Tanner AC, Haffajee AD, Socransky SS. Gram negative species associated with active destructive peri- odontal lesions. J Clin Periodont 1985; 12: 648-659. 30. Ebersole JL. Systemic humoral immune responses in
periodontal disease. Crit Rev Oral Biol Med 1990: 1:
283-331.
31. Everett FG, Kramer GM. The disto-lingual groove in the maxillary lateral incisor: a periodontal hazard. 1 Peri- odontol 1972: 43: 352-361.
32. Feldman RS, Bravacos JS, Rose CL. Association between smoking differenr tobacco products and periodontal dis- ease indexes. J Periodontol 1983: 54: 481-487.
33. Finley BB, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev 1989: 53: 210-230.
34. Freter R, Brickner H, Botney M, Cleven D, Aranki A. Mechanisms that control bacterial populations in con- tinuous-flow culrure models of mouse large intestinal flora. Infect Immun 1983: 39: 676-685.
35. Geiger AM, Wasserman BH, Thompson RH Jr, Turgeon LR. Relationship of occlusion and periodontal disease. V.
Relation of classification of occlusion to periodontal sta- tus and gingival inflammation. J Periodontol 1972: 43: 554-560.
36. Geiger AM, Wasserman BH, Turgeon LR. Relationship of occlusion and periodontal disease. VIII. Relationship of crowding and spacing to periodontal destruction and gin- gival inflammation. J Periodontol 1974: 45: 43-49. 37. Genco RJ, Zambon JJ. Clinical microbiology in the diag-
nosis and treatment of periodontal disease. J Am Coll Dent 1989: 56: 19-27.
38. Gilrnore N, Sheiham A. Overhanging dental restorations and periodontal disease. J Periodontol 1971: 42: 8-12. 39. Glickman I, Petralis R, Marks R. The effect of powered
toothbrushing and interdental stimulation upon micro- scopic inflammation and surface keratinization of the interdental gingiva. J Periodontol 1965: 36: 108-11 1.
40. Glickman I, Smulow J. Effect of excessive occlusal forces upon the pathway of gingival inflammation in humans. J Periodontol 1965: 36: 141-147.
41. Gmur R, Guggenheim B. Antigenic heterogeneity of
Bacteroides intermedius as recognized by monoclonal antibodies. Infect Immun 1983: 42: 459-470.
42. Goldman HM. Development of physiologic gingival con- tours by gingivoplasty. Oral Surg Oral Med Oral Pathol 43. Goldschmidt PR, Logen RB, Taubman SB. Effects of amal- gon corrosium products o n human cells. J Periodont Res 44. Goodson JM, Tanner AC, Haffajee AD, Sornberger GC, Socransky SS. Patterns of progression and regression of
advanced destructive periodontal diseases. J Clin Peri- odontol 1982: 9: 472-481.
45. Gould MS, Picton DC. The relation between irregularities
of teeth and periodontal disease. Br Dent J 1966: 121: 20-23.
46. Grbic JT, Lamster IB. Risk indicators for future clinical attachment loss in adult periodontitis. Tooth and site variables. J Periodontol 1992: 63: 262-269.
47. Greene JC. Periodontal disease in India: report of a n epi- demiological study. J Dent Res 1960: 39: 302-312. 26: 218-229.
The role of local factors ~~ ~
48. Greene JC. Oral hygiene and periodontal disease. Am J Public Health 1963: 53: 913-922.
49. Greenstein G, Caton J. Periodontal disease activity: a criti- cal assessment. J Periodontol 1990: 61: 543-552. 50. Grenier D, Mayrand D. Selected characteristics of patho-
genic and nonpathogenic strains of Bacteroides gingivalis.
J Clin Microbiol 1987: 25: 738-740.
51. Haffajee AD, Socransky SS, Goodson JM. Clinical par- ameters as predictors of destructive periodontal disease activity. J Clin Periodontol 1983: 10: 257-265.
52. Haffajee AD, Socransky SS, Ebersole JL, Smith DJ. Clinical, microbiological, and immunological features associated with the treatment of active periodontosis lesions. J Clin Periodontol 1984: 11: 600-618.
53. Haffajee AD, Socransky SS, Lindhe J, Kent RL, Okamoto H, Yoneyama T. Clinical risk indicators for periodontal attachment loss. J Clin Periodontol 1991: 18: 117-125. 54. Haffajee AD, Socransky SS, Smith C, Dibart S. Relation
of baseline microbial parameters to future periodontal attachment loss. J Clin Periodontol 1991: 18: 744-750. 55. Haffajee AD, Socransky SS, Smith C, Dibart S. Microbial
risk indicators for periodontal attachment loss. J Peri- odont Res 1991: 26: 293-296.
56. Han N, Hoover CI, Winkler JR, Ng CY, Armitage GC. Identification of genomic clonal types of Actinobacillus actinomyceteincomitans by restriction endonuclease analysis. J Clin Microbiol 1991: 29: 1574-1578.
57. Haney JM, Leknes KN, Lie T, Selvig KA, Wilkesjo UM. Cementa1 tear related to rapid periodontal breakdown: a case report. J Periodontol 1992: 63: 220-224.
58. Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingiualis in nonhuman pri- mates initiates progression of periodontitis. Science 1988: 59. Holt SC, Bramanti TE. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med 1991: 2: 177-281.
60. Ingervall B. A clinical study of the relationship between crowding of teeth, plaque, and gingival condition. J Clin Periodontol 1977: 4: 214-222.
61. Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP. Responses of four tooth and site groupings to periodontal therapy.
J Periodontol 1990: 61: 173-179.
62. Kalkwarf KL, Reinhardt RA. The furcation problem and current controversies and future directions. Dent Clin North Am 1988: 22: 243-266.
63. Kirkegaard E, Borgnakke WS, Grnbaek L. Dental diseases, treatment needs and dental care habits in a representa- tive segment of the adult Danish population. Tandlaege- bladet 1987: 91: 1-36.
64. Kornman KS, Loesche WJ. Effects of estradiol and pro- gesterone on Bacteroides melnninogenicus and Bacter- oides gingiualis. Infect Immun 1982: 35: 256-263. 65. Kornman KS, Robertson PB. Clinical and microbiological
evaluation of therapy for juvenile periodontitis. J Peri- odontol 1985: 56: 443-446.
66. Kornman KS, Newman MG, Choi J-I. Effects of smoking on the clinical and microbial outcomes of periodontal therapy. In preparation.
67. Lamster IB, Celenti R, Ebersole JL. The relationship of serum IgG antibody titers to periodontal pathogens to indicators of the host response in crevicular fluid. J Clin Periodontol 1990: 17: 419-425.
239: 55-57.
68. Lang NP, Kiel RA, Anderhalden K. Clinical and microbio- logical effects of subgingival restorations with over- hanging or clinically perfect margins. J Clin Periodontol
69. Larato DC. Relationship of food impaction to intrabony lesions. J Periodontol 1971: 42: 237-238.
70. Lee KW, Lee EC, Peon KY. Palato-gingival grooves in maxillary incisors. A possible predisposing factor to local- ized periodontal disease. Br Dent J 1968: 124: 14-18. 71. Lembariti BS, Frencken JE, Pilot T. Prevalence and sever-
ity of periodontal conditions among adults in urban and rural Morogoro, Tanzania. Community Dent Oral Epide- miol 1988: 16: 240-243.
72. Lindhe J, Haffajee AD, Socransky SS. Progression of peri- odontal disease in adult subjects in the absence of peri- odontal therapy. J Clin Periodontol 1983: 10: 433-442. 73. Lindhe J, Okamoto H, Yoneyama T, Haffajee A, Socransky
SS. Longitudinal changes in periodontal disease in un- treated subjects. J Clin Periodontol 1989: 16: 662-670. 74. Lindhe J, Okamoto H, Yoneyama T, Haffajee A, Socransky
SS. Periodontal loser sites in untreated adult subjects. J Clin Periodontol 1989: 16: 671-678.
75. Listgarten MA, Ellegaard B. Electron microscopic evi- dence of a cellular attachment between junctional epi- thelium and dental calculus. J Periodont Res 1973: 8 :
76. Listgarten MA, Mayo HE, Tremblay R. Development of dental plaque on epoxy resin crowns in man. A light and electron microscope study. J Periodontol 1975: 46: 10-26. 77. Littleton NW. Dental caries and periodontal diseases among Ethiopian civilians. Public Health Rep 1963: 78:
78. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 1963: 21: 79. Loe H, Theilade E, Jensen SB, LOC H. Experimental gingi-
vitis in man. Part 11. J Periodontol 1965: 36: 177-187. 80. Loe H. Reactions of marginal periodontal tissues to res-
toration procedures. Int Dent J 1968: 18: 759-778.
81. Loe H, Schiott CR, Karring G, Karring T. Two years oral use of chlorhexidine in man. I. General design and clinical effects. J Periodont Res 1976: 11: 35-44.
82. Loe H, Anerud A, Boysen H, Smith M. The natural history of periodontal disease in man. The rate of periodontal destruction before 40 years of age. J Periodontol 1978: 83. Loe H, Anerud A, Boysen H, Morrison E. Natural history
of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol 1986: 13: 431-445.
84. Loesche WJ. Importance of nutrition in gingival crevice microbial ecology. Periodontics 1968: 6: 245-249. 85. Loesche WJ, Gusberti F, Mettraux G, Higgins T, Syed S.
Relationship between oxygen tension and subgingival bacterial flora in untreated human periodontal pockets. Infect Immun 1983: 42: 659-667.
86. Loesche WJ, Syed SA, Schmidt E, Morrison EC. Bacterial profiles of subgingival plaques in periodontitis. J Peri- odontol 1985: 56: 447-456.
87. Loos BG, Mayrand D, Genco RJ, Dickinson DP. Genetic heterogeneity of Porphyromonas (Bacteroides) gingiualis
by genomic DNA fingerprinting. J Dent Res 1990: 69:
1488-1493. 1983: 10: 563-578.
143-150.
631-640. 533-55 1 .
Kornman & Loe 88. 89. 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100, 101. 102. 103. 104. 105. 106. 10;. 108.
Lovdal A, Arno A, Waerhaug I. Evidence of clinical mani- festations of periodontal disease in light of oral hygiene and calculus formation. J Am Dent Assoc 1958: 56: 2 1-33. MacGregor I, Edgar W M , Greenwood AR. Effects of ciga- rette smoking on the rate of plaque formation. J Clin Peri- odontol 1985: 12: 3 5 4 1 .
Mardani-Bey W, Majzoub Z, Kon S. Anatomic consider- ations in the etiology and management of maxillary and mandibular molars with furcation involvement. Int J Peri- odont Restorative Dent 1991: 11: 399-409.
Markitzin A. A ten year follow-up study of alveolar bone lcss influenced by two dissimiliar class I1 amalgam res- torations. J Oral Rehabil 1987: 14: 23-25.
Marsh PD, McKee AS, McDermid AS, Dowsett AB. Ultra- structure and enzyme activities of a virulent and an avi- rulent variant of Bacteroides gingiznlis W50. FEMS Micro- biol Lett 1989: 50: 181-185.
Marshall-Day CD, Stephens RG. Quigley LF Jr. Peri- odontal disease: prevalence and incidence. J Periodontol Master DH, Hoskins SW. Projections of cervical enamel on molar furcations. J Periodontol 1964: 35: 49-53. Maurelli AT. Temperature regulation of virulence genes in pathogenic bacteria: a general strategy for human pathogens? Microb Pathog 1989: 7: 1-10,
McKee AS, McDermid SS. Baskerville A, Dowsett AB, Ell- wood DC. Effect of hemin on the physiology and viru- lence of Bacreroides gingiznlis W50. Infect Immun 1986: McKee AS. McDermid AS, LVait R. Baskerville A, Marsh PD. Isolation of colonial variants of Bacteroides girzgiinlisW50 with a reduced virulence. J Med Microbioll988: 27: 59-64. Michalowicz BS, Aeppli D, Virag JG et al. Periodontal findings in adult twins. J Periodontol 1991: 62: 293-299. Michalowicz BS. Aeppli DP. Kuba RK et al. A nvin study of genetic variation in proportional radiographic alveolar bone height. J Dent Res 1991: 70: 1431-1435.
Miller AJ, Brunelle JA, Carlos JP, Brown L], Loe H. The national survey of oral health in U.S. employed adults and seniors: 1985-1986. Bethesda, MD: NIH Publication Miller JF, Mekalanos JJ. Falkow SF. Coordinate regulation and sensory transduction in the control of bacterial viru- lence. Science 1989: 243: 916-922.
Mombelli A, McNabb H, Lang NP. Black-pigmenting gram-negative bacteria in periodontal disease. I. Topo- graphic distribution in the human dentition. J Peri- odontol Res 1991: 26: 301-307.
Moore WEC, Holdeman LV, Smibert RM et al. Bacter- iology of experimental gingivitis in young adult humans. Infect Immun 1982: 38: 651-667.
Mundy GR. Inflammatory mediators and the destruction of bone. J Periodontol Res 1991: 26: 213-217.
Musser JM, Kroll JS, Moxon ER, Selander RK. Evolutionary genetics of the encapsulated strains of Haenzoplzihts i n -
f!urrzzne. Proc Natl Acad Sci 1988: 85: 7758-7762. Neiders M E , Chen PB, Suido H, Reynolds HS. Zanibon JJ. Heterogenicity of virulence among strains of Bacteroides
gingifdis. J Periodontol Res 1989: 24: 192-198.
Newman MG. Socransky SS. Savitt ED, Propus DA, Craw-
ford A. Studies of the microbiology of periodontosis. J Periodontol 1976: 47: 373-379.
Ochsenbein C, Ross SE. A reevaluation of osseous surger).. Dent Clin North Am 1969: 12: 87-102.
1955: 26: 185-203.
52: 349-355.
NO. 87-2868. August 1987.
109. 110. 111. 112. 113. 114. 115. 116. 117. 118. 119. 120. 121. 122. 123. 124. 125. 126. 127. 128. 129. 130.
O'Leary T, Badell MC. Bloomer PS. Interproximal contact and marginal ridge relationships in periodontally healthy young males classified as to orthodontic status. J Peri- odontol 1975: 46: 6-9.
Oshrain HI, Salkind A, Mandel ID. A histologic compari- son of supra- and subgingival plaque and calculus. J Peri- odontol 1971: 42: 31-33.
Page RC, Schroeder H. Periodontitis in man and other animals. A comparative review. Basel: S. Karger, 1982. Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodont Res Papapanou PN, Wennstrom JL, Grondahl K. Periodontal status in relation to age and tooth type. A cross-sectional radiographic study. J Clin Periodontol 1988: 15: 469- 478.
Papapanou PN, Wennstrom JL. A 10-year retrospective study of periodontal disease progression. Clinical charac- teristics of subjects with pronounced and minimal dis- ease development. J Clin Periodontol 1990: 17: 78-84. Parfitt GJ. A survey of oral health of Navajo Indian children. Arch Oral Biol 1960: 1: 193-205.
Patters MR, Landesberg RL, Johansson LA, Trummel CL, Robertson PB. Bacteroides gingiualis antigens and bone resorbing activity in root surface fractions of periodont- ally involved teeth. J Periodont Res 1982: 17: 122-130. Preber H, Bergstrom J. The effect of non-surgical treat- ment on periodontal pockets in smokers and non- smokers. J Clin Periodontol 1986: 13: 319-323.
Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodontol 1959: 30: 51-59. Ramfjord SP. Bruxism, a clinical and electromygraphic study. J Am Dent Assoc 1961: 62: 21-44.
Russell AL. A social factor associated with the severity of periodontal disease. 1 Dent Res 1957: 36: 922-926. Russell AL. Some epidemiological characteristics of peri- odontal disease in a series of urban populations. J Peri- odontol 1957: 28: 286-293.
Schluger S. Osseous resection - a basic principle in peri-
odontal surgery. Oral Surg Oral Med Oral Pathol 1949: 2: 316-325.
Schroeder HE. Formation and inhibition of dental calcu- lus. J Periodontol 1969: 40: 643-646.
Schroeder HE. The effects of furcation morphology on periodontal disease. Zahnarztliches Institut der Universi- tat Zurich. Dtsch Zahnarztl Z 1991: 46: 324-327.
Schwartz J, Weiss ST. Host and environmental factors in- fluencing the peripheral blood leukocyte count. Am J Epi- demiol 1991: 134: 1402-1409.
Shah HN, Seddon SV, Gharbia SE. Studies on the viru- lence properties and metabolism of pleiotropic mutants of Porphyromonas gingimlis (Bacteroides girgigivalis) W50. Oral Microbiol Immunol 1989: 4: 19-23.
Silness I. Periodontal conditions in patients treated with dental bridges. 1 Periodont Res 1974: 9: 50-55.
Silness J, Roystrand T. Relationship between alignment conditions of teeth in anterior segments and dental health. J Clin Periodontol 1985: 12: 312-320.
Slots J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res 1976: 84: 1-10,
Slots J, Listgarten MA. Bacteroides gingiualis, Bacteroides internzedius and Actinobacillirs actinoniycetemcornitans
in human periodontal diseases. J Clin Periodontol 1988: 15: 85-93.