ISSN: 1040-8363 print / 1549-781X online DOI: 10.1080/10408360701295623
ANTI-CITRULLINE ANTIBODIES IN THE DIAGNOSIS AND PROGNOSIS OF RHEUMATOID ARTHRITIS: Evolving Concepts
Amalia Raptopoulou, Prodromos Sidiropoulos, Maria Katsouraki, and Dimitrios T. Boumpas 2 Department of Internal Medicine and Division of Rheumatology, Clinical Immunology and Allergy, University of Crete, Medical School, Heraklion, Greece
Referee Dr. Pierre Geborek, Department of Rheumatology, Lund University Hospital, Lund, Sweden
2 Citrulline is a non-standard amino acid that can be incorporated into proteins only by post-translational modification of arginine by peptidylarginine deiminase (PAD) enzymes during a variety of biologic processes, including inflammation. Rheumatoid arthritis (RA) is an inflammatory au-toimmune disease, with a prevalence of 0.3 to 1% worldwide, which leads to progressive joint erosion and substantial disability if not treated early. A reliable and specific test for a marker present early in the disease would be useful to identify RA patients prior to the occurrence of joint damage. A new group of autoantibodies, the anti-cyclic citrullinated peptide antibodies (anti-CCP), can be detected in up to 80% of patients with RA, are highly specific for the disease, and may be of value for both the diagnosis and the prognosis of RA. The fact that these antibodies may appear before the onset of the disease suggests a potential role in primary prevention. Interestingly, they may also play a role in the pathophysiology of this disabling disease. The process of citrullination, its physiologic role, and citrullination-related pathologies, as well as the use of anti-citrullinated protein antibody tests (ACPA) for the early diagnosis and prognosis of RA and their potential role in the pathophysiology of the disease, are discussed.
Keywords Anti-cyclic citrullinated peptide antibodies, antiperinuclear factor, autoan-tibodies, citrulline, diagnosis, early arthritis, rheumatoid arthritis, primary prevention, prognosis
Abbreviations ACPA,anti-citrullinated protein antibodies;AFA,anti-filaggrin antibod-ies;AhFibA,anti-human fibrin(ogen) autoantibodies;AKA,anti-keratin antibodies; anti-CCP,anti-cyclic citrullinated peptide antibodies;anti-Sa,anti-citrullinated vimentin an-tibodies;anti-TNFα,anti-tumor necrosis factor-αantibodies; APF,antiperinuclear fac-tor;CCP,cyclic citrullinated peptide;CK,cytokeratin;DMARDS,disease-modifying anti-rheumatic drugs;H2A, H3, and H4,histone core proteins;HCV,hepatitis C virus; HL-60,a promyelocytic cell line;HLA-DR,human class II histocompatibility antigen;JIA,
Address correspondence to Dr. Dimitrios T. Boumpas, University of Crete, Medical School, PO Box 2208, 71003 Heraklion, Greece. E-mail: boumpasd@med.uoc.gr
juvenile idiopathic arthritis;MBP,myelin basic protein;MHC,major histocompatibility complex;OR,odds ratio;PAD,peptidylarginine deiminase;RA,rheumatoid arthritis;
RF,rheumatoid factor;Sa,citrullinated vimentin (vimentin is an intermediate filament protein);SE,shared epitope;SLE,systemic lupus erythematosus;SNPs,single nucleotide polymorphisms;SS,Sjogren syndrome
I. INTRODUCTION
Rheumatoid arthritis (RA) is a common autoimmune disease with a prevalence of up to 1% of the population worldwide. Patients with RA follow a variable disease course and have different prognoses with regard to func-tional status and overall survival. Therapeutic approaches for RA have greatly improved during the last decade. Treatment at an early phase of the disease with disease-modifying anti-rheumatic drugs (DMARDS) improves the dis-ease course and halts the damage to the joints. New biologic agents (anti-tumor necrosis factor-αantibodies [anti-TNFα], B-cell depleting agents, and inhibitors of co-stimulation), when used in combination with DMARDS, of-fer greater rates of efficacy and a more effective inhibition of radiographic progression compared to that observed with any of the known combinations of DMARDS.
RA is accompanied by the presence of many autoantibodies. While most of these autoantibodies, like rheumatoid factor (RF), are not specific for RA, some appear to have higher specificity and are, in some cases, almost exclusively present in RA. A new era began when analyses identifying anti-citrullinated protein antibodies (ACPA) were introduced for the diagnosis of arthritis. ACPA constitute a growing family of autoantibodies, in which the first member, antiperinuclear factor (APF), was described over 40 years ago. The major breakthrough, however, came with the realization of the crucial role of peptide epitopes, which were citrullinated post-translationally, and the subsequent development of the anti-cyclic citrullinated peptide antibodies (anti-CCP) test. In this review, we begin with a discussion of the process of citrullination, its physiologic role, and citrullination-related pathologies. Next, we review critically the use of the ACPA tests for the early diagnosis and prognosis of RA and for their potential role in the pathophysiology of the disease.
II. CITRULLINE AND CITRULLINATION: REGULATION AND PATHOLOGIES
A. Citrulline and Citrullination
1. Origin of Citrulline
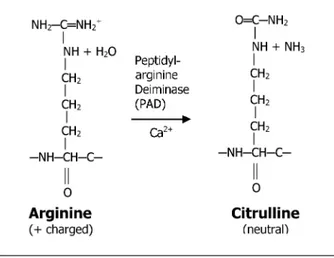
FIGURE 1 The enzymatic conversion of protein-bound arginine to citrulline. Citrullination is the post-translational modification of protein-bound arginine into the non-standard amino acid citrulline by a specific enzyme, PAD, in the presence of sufficient concentrations of Ca2+. Citrullinated proteins differ from their non-modified forms in their electrophoretic mobility because of changes in their charge as well as their conformation. Citrullination may have a significant impact on intramolecular and intermolecular interactions of the target proteins and plays an important role during apoptosis. More importantly, citrul-lination may alter the immunologic recognition by antibodies and thus play a crucial role in immunologic response and control.
encoded by DNA or RNA. The most significant difference between arginine and citrulline is that arginine is one of the most basic amino acids while cit-rulline lacks any charge.2Citrullination is deimination by a specific enzyme, protein-arginyl deiminase (PAD, EC 3.5.3.15) (Figure 1)3.
The metabolism of citrulline, a member of the urea cycle, is tightly regu-lated. It occurs in two forms, free and bound. Metabolism of protein-bound citrulline is independent of metabolism of the free form. Free cit-rulline metabolism involves three key enzymes: NO synthase (NOS), which converts arginine to citrulline and NO; ornithine carbamoyltransferase (OCT), which catalyses the conversion of ornithine into citrulline; and argini-nosuccinate synthetase (ASS), which converts citulline into argininosucci-nate. The tissue distribution of these enzymes indicates three metabolic pathways for citrulline with distinct pathologies (see below).
2. Citrullination-Mediating Enzymes
motif, and they depend on high concentrations of Ca2+for their enzymatic activity.2
Normally, PAD are present intracellularly as inactive enzymes. The intra-cellular calcium concentration in normal cells (0.1µmol/l) is much lower than the threshold calcium concentration for PAD activity (approximately 10 µmol/l).3 Because the required concentration of Ca2+ is much higher than the cytosolic concentration, conversion of arginine to citrulline residues appears to be carried out either in a microenvironment, where extraordinar-ily high concentrations of Ca2+are achieved in tightly regulated conditions,
or in an extracellular environment, where there is leakage of enzyme from dying cells.3
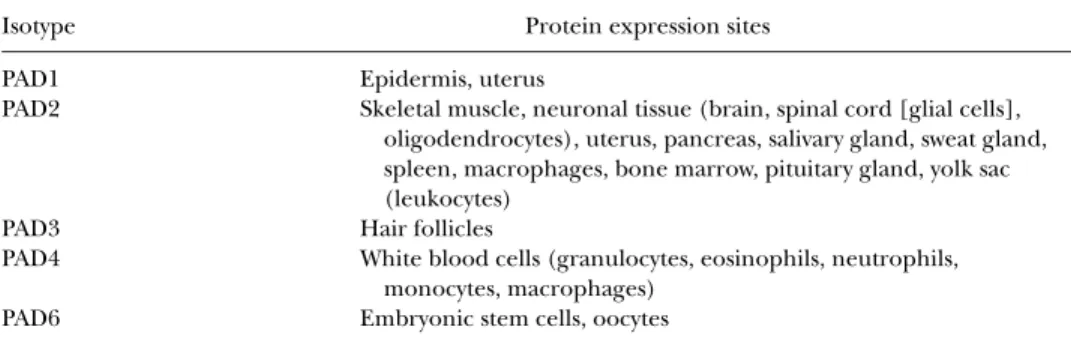
All known natural substrates of PAD are proteins that have important structural functions. Citrullination may have serious consequences for in-tramolecular and intermolecular interactions of the target protein. The main difference among the isotypes is their tissue-specific expression profile.4The differences in tissue distribution among PAD isotypes are expected to be re-lated to their physiologic functions (Table 1).2
3. Regulation of PAD Activity
The current knowledge of the molecular and enzymatic features of PAD is limited. The majority of reported biochemical data were obtained using PAD2 from rat skeletal muscle.5
The activity of PAD2 was shown to be completely dependent on calcium ions. Half-maximal activity was observed at 40–60µmol/l Ca2+, and calcium ions could not be replaced by other divalent cations without loss of activity. A sequence comparison of rat PAD2 and calcium-binding domains of vari-ous proteins, including intestinal calcium-binding protein and calmodulin, identified a putative calcium-binding site between residues 486 and 524.6
However, calcium-binding studies showed that one molecule of PAD protein can bind three Ca2+ions, suggesting that there is more than a single
calcium-binding site per molecule. Addition of phosphatidylserine and 1,2-diolein to the reaction medium reduced the calcium concentration for half-maximal
TABLE 1 Tissue Distribution of PAD Enzymes4
Isotype Protein expression sites
PAD1 Epidermis, uterus
PAD2 Skeletal muscle, neuronal tissue (brain, spinal cord [glial cells], oligodendrocytes), uterus, pancreas, salivary gland, sweat gland, spleen, macrophages, bone marrow, pituitary gland, yolk sac (leukocytes)
PAD3 Hair follicles
PAD4 White blood cells (granulocytes, eosinophils, neutrophils, monocytes, macrophages)
activity from 40–60 to 15–20 µmol/l, suggesting that PAD2 activity might preferentially take place in a membranous environment. Moreover, PAD2 activity is enhanced in a reducing environment, indicating that PAD activity can be upregulated by modulation of disulfide bonds. Optimal PAD2 activity was observed at pH 7.5 and at 50◦C in the presence of 10 mmol/l CaCl2and
2 mmol/l dithiothreitol.
Steroid hormones and aging appear to affect PAD expression in rat and mouse, although expression differences are much more pronounced at the mRNA than at the protein level. Evidence for the regulation of PAD expres-sion at the translational level comes from studies addressing PAD expresexpres-sion during human monocyte differentiation into macrophages, where a signifi-cant increase in PAD2 protein level is observed, although PAD2 mRNA levels remain virtually unchanged.7
During differentiation of HL-60 cells (a promyelocytic cell line) to granu-locytes or monocytes following treatment with all-trans retinoic acid or 1α ,25-dihydroxyvitamin D3, respectively, PAD expression and activity increased, strongly suggesting that myeloid cell differentiation induces PAD expression. Indeed, activation of PAD has been seen in human and mouse macrophages, but only upon treatment with the calcium ionophore ionomycin. Treatment of cells with this ionophore results in calcium influx from the extracellular environment, subsequent activation of PAD, and citrullination of proteins including vimentin (an intermediate filament protein). When vimentin be-comes citrullinated, the cytoskeleton collapses and falls back to the peri-nuclear region, one of the events occurring early in apoptosis. This suggests that citrullination of structural proteins may initiate major changes in cell morphology.
In conclusion, PAD activity increases during (terminal) differentiation of cells, as seen in keratinocytes and monocytes, or by induction of apoptosis, in the case of granulocytes. During these processes, activation of PAD is induced by elevation of intracellular calcium levels or leakage of the enzyme into the extracellular space as a result of cell membrane disruption.4
B. Physiologic Function and Effects of Citrullination on Protein Structure
its natural substrate trichohyalin (THH). Citrullination of THH facilitates its association with cytokeratins and leads to cross-linkage of keratin filaments. This, in turn, results in the formation of rigid structures that ultimately facil-itate hair fiber growth.8
The most widely expressed isotype, PAD2, is present in many different tis-sues. Despite this broad expression pattern, only myelin basic protein (MBP), an abundant protein of the myelin sheath whose citrullination occurs dur-ing development of the central nervous system,9 and vimentin have been
identified as natural substrates of PAD2. Citrullination of vimentin was ob-served during calcium-ionophore induced apoptosis of mouse peritoneal macrophages. Recently, citrullinated vimentin was shown to be the target of the RA-specific anti-citrullinated vimentin antibodies (anti-Sa).
PAD4 isotype is localized in the nucleus, in contrast to the PADs dis-cussed above, which are all localized in the cytoplasm. A putative monopar-tite motif, found in the N-terminal part of human PAD4, has recently been shown to be essential for the nuclear localization of the protein.4 PAD4 is expressed mainly in white blood cells, granulocytes10and monocytes,7 and
can therefore be detected in a variety of tissues.11−13 Stimulation of PAD4-expressing granulocytes with a calcium ionophore induces the citrullination of proteins.6Likewise, HL-60 cells from promyelocytic cell line showed
spe-cific citrullination of nuclear proteins when differentiated into granulocyte-like cells after ionophore treatment.12Substrates of PAD4 in the nucleus are
histone core proteins (H2A, H3, and H4) and nucleophosmin/B23, a nucleo-lar protein that functions in ribosome assembly, nucleocytoplasmic transport, and centrosome duplication.4,14−16Although no data exist on the function of histone citrullination, one might speculate about a role in apoptosis.1,2 Finally, data concerning expression of PAD6 in leukocytes, as demonstrated by real time-polymerase chain reaction (RT-PCR), need to be further verified at the protein level.4,17
In summary, although some biological events, such as inflammation, apoptosis, trauma, and aging, increase post-translational citrullination, the precise physiologic role of citrullination is still unknown.2 What is clear at present is that a variety of proteins are citrullinated and subsequently change their conformation. Because the enzymes are only active in high concentra-tions of Ca2+, citrullination should occur outside cells or in a tightly reg-ulated intracellular environment. Citrullinated proteins differ from their non-modified forms in their electrophoretic mobility because of changes in their charge as well as their conformation. The biochemical changes caused by citrullination resemble denaturing proteins with detergents.18
peptides increases peptide-major histocompatibility complex (MHC) affin-ity and activates CD4+T cells in human class II histocompatibility antigen (HLA-DR4) transgenic mouse.20This finding supports the idea of alteration
of antigenicity by citrullination; moreover, it implies that a change in anti-genicity by peptidyl citrullination may have a role in the context of HLA-DR4-dependent antigen recognition.2
C. Citrullination-Related Pathologies
Intracellular calcium levels under normal physiological conditions are insufficient for PAD activity. This suggests a relationship between PAD ac-tivity and terminal differentiation, or death of cells, where control of cal-cium homeostasis is lost. The citrullination of filaggrin and keratin dur-ing terminal differentiation of keratinocytes and the citrullination of vi-mentin and histones during apoptosis support this idea. During cell death, the integrity of the plasma membrane is lost,6,11 causing influx of cal-cium from the extracellular space and subsequent activation of intracellu-lar PAD.12,14−17Alternatively, PAD may leak out of dying cells, become acti-vated (the extracellular calcium concentration is approximately 1 mmol/l, which is sufficient for PAD activity13), and cause citrullination of extracellular
proteins.
The physiological roles of PAD in cells and tissues, however, have yet to be fully determined. PAD and citrullinated proteins are associated with the human diseases psoriasis, multiple sclerosis, and RA, as well as with various tumors. Psoriasis is a chronic skin disease characterized by the absence of cit-rullinated keratin K1 in the epidermis.21,22Multiple sclerosis is characterized by highly citrullinated forms of MBP that are present in the affected area of the brain and seem to be involved in the demyelination of neurons.9
PAD4 is significantly expressed and is co-located with cytokeratin (CK, a well-known tumor marker) in various tumors, especially adenocarcinoma, suggesting that the enzyme may be a valuable tumor marker for clinical and experimental oncology. The citrullination of CK in the tumors and the resistance of citrullinated CK to caspase-mediated cleavage support PAD4 involvement in the disruption of the apoptotic process in tumor cells.23
RA is characterized by large numbers of autoantibodies, some of which are directed against citrullinated proteins and are produced when citrulline residues in proteins become recognized as a major epitope of autoantigens. Recently, it has been shown that PAD4 is highly expressed in RA synovial tissue and locally citrullinates fibronectin.24Citrullination could, in turn, al-ter inal-teractions with its receptors and with growth factors and, consequently, contribute to mechanisms of RA pathogenesis, such as perturbed angiogen-esis and apoptosis.
III. RA
RA is characterized by the destruction of multiple joints along with mul-tiple organ involvement. Highly complex pathways of tissue damage and ineffective repair are unquestionably involved in creating and maintaining the lesion that harms cartilage and bone (Figure 2).25
A. RA-Associated Autoantibodies
Although various autoantigens have been proposed as targets of pathogenic T cells and B cells in RA, none of their pathogenic mechanisms or significance has been confirmed. Cellular immune mechanisms have also been implicated, as many T cells and antigen-presenting cells are present in RA synovial tissues. RA synovial T cells are known to be activated, and the HLA-DRB gene has been shown to be strongly associated with the disease. Evidence also supports the involvement of B cells that, in the context of RA synovium, show oligoclonality, differentiate into plasma cells, and produce autoantibodies.
1. Rheumatoid Factor (RF)
RF is an antibody directed to the Fc domain of IgG molecules and is present in approximately 75% of RA patients. However, it can also be detected in other autoimmune and infectious diseases, and in 3–5% of the healthy population, a proportion that increases to 10–30% in the elderly. Thus, it has a low specificity for RA.4
2. Anti-Citrullinated Protein Antibodies (ACPA)
In addition to RF, several autoantibodies have been reported to be more specific and to have higher positive predictive values for RA. Some of these highly RA-specific autoantibodies have been found to recognize citrullinated peptides and thus are called anti-citrullinated protein antibodies (ACPA).2
3. Antiperinuclear Factor Autoantibodies (APF)
ACPA were first described in 1964 by two Dutch scientists who showed that RA sera reacted with an unknown protein scattered around the perinuclear region of human buccal epithelial cells; they called it perinuclear factor.26,27
4. Anti-Keratin Antibodies (AKA)
Many years later, when rat esophagus tissue was used as substrate, Young
et al.28described a specific staining of filaments in the stratum corneum of the epithelium. Assuming that keratin was the antigen, they called these anti-bodies AKA (anti-keratin antianti-bodies). In RA, APF and AKA have a sensitivity of 43–52% and a specificity of 97–99%.
5. Anti-Filaggrin Antibodies (AFA)
Further experiments established that both APF and AKA reacted with filaggrin, a citrulline-containing protein associated with keratin filaments.29 These antibodies have since been referred to as anti-filaggrin antibodies (AFA). The reactivity of AFA was subsequently shown to be absolutely depen-dent on the citrullination of the filaggrin protein.
6. Anti-Sa
Another RA-specific antibody system, anti-Sa, that also belongs to the fam-ily of ACPA, was originally described in 1994.30The antibodies, which can be
detected by immunoblotting using extracts of placenta or RA pannus tissue, could be detected in 43% of RA patients with high specificity (99%).31These
findings were subsequently confirmed by several independent studies.32
B. Citrullinated Proteins in RA Joints
1. Filaggrin
Filaggrin was first identified as a citrullinated self-peptide recognized by RA-specific sera. It is produced as profilaggrin (400 kDa) in the late stage of skin differentiation and is stored in keratohyaline granules of keratinocytes. Profilaggrin is a phosphorylated protein with 10–12 repeated motifs of 324 amino acids (filaggrin unit), which is dephosphorylated and cleaved during keratinization to become filaggrin. Furthermore, arginine residues of filag-grin are converted to citrulline by PAD. These citrulline residues are impor-tant in epitopes recognized by RA autoantibodies. Additional investigations revealed that plasma cells produced antibodies to citrullinated filaggrin in the joint at the site of inflammation. Filaggrin, however, is not a component of the joint; citrullinated filaggrin, the target of the specific RA autoanti-bodies detected in the APF, AKA, and AFA tests, is not expressed in synovial joints.29,33
2. Fibrin
Citrullinated fibrin is efficiently recognized by autoantibodies present in RA sera (anti-human fibrin(ogen) autoantibodies, AhFibA). Indeed, citrul-linated forms of the α- andβ-chains of fibrin have been reported to occur in the inflamed synovium. The presence of citrullinated fibrin during joint inflammation has been also confirmed in chronic and acute mouse mod-els for arthritis. In the inflamed synovium, oxygen metabolism, which is in disequilibrium, leads to sites of hypoxia where plaques containing extravas-cular fibrin are commonly found. Such deposits also contain citrullinated proteins, one of which has been identified as citrullinated fibrin. It seems, however, that fibrin deimination in the inflamed synovium is a general phe-nomenon associated with any synovitis and is therefore not a specific feature for RA.34
3. Vimentin
It has been known for some time that the Sa antigen, which is specifi-cally targeted by autoantibodies in RA sera, is also present in inflamed syn-ovium. Analysis of the molecular identity of this antigen showed that Sa is citrullinated vimentin. Monocyte-derived macrophages have been shown to contain citrullinated vimentin. Because macrophages are abundant in the RA synovium, it is not surprising that citrullinated vimentin is present in the synovium as well.35
4. Histones
Citrullination of histones has been described ex vivo during calcium-ionophore induced apoptosis of granulocytes.16,17Because large numbers of
the site of inflammation, which may trigger histone citrullination. Although the presence of citrullinated histones in the inflamed synovium has not yet been reported, it is not unlikely that they are present.
5. Other Proteins
It is likely that additional citrullinated autoantigens are produced in an inflamed environment. PAD4 has been reported to be activated in dy-ing granulocytes, and, as a consequence, several nuclear proteins become citrullinated.36When such apoptotic cells are not cleared efficiently, the cit-rullinated proteins might be exposed to the immune system, eventually lead-ing to the production of ACPA. In addition to these citrullinated proteins, citrullinating PAD has also been detected in inflamed joints.37
C. Genetic and Environmental Links with Citrullination in RA
Besides citrullination, many factors, both environmental and genetic, have been investigated for their involvement in RA. Some appear to be linked to citrullination of proteins. Among them are genes encoding certain antigen-presenting molecules and single nucleotide polymorphisms (SNPs) in genes encoding PAD4. Interestingly, smoking was recently shown to be a risk factor for positive anti-CCP and positive RF in the presence of shared epi-tope (SE) in patients with RA; thus, an environmental stimulus may induce mechanisms that, in a specific genetic context, can cause the emergence of arthritis.38,39
1. HLA-DRB1 SE
TheHLA-DRB1product SE, a common region of highly similar sequence among certain HLA-DRclass II alleles, is the best-known genetic factor as-sociated with RA. It has been mapped to the third hypervariable region of DR chains, especially amino acids 70–74, encoding a conserved amino acid sequence (QKRAA, QRRAA, or RRRAA) that forms the fourth anchor-ing pocket in the HLA groove. This common epitope is found in multiple RA-associated DR molecules, includingDR4, DR1, andDR14.MHC class II molecules expressing SE can bind and present citrullinated peptides to T cells. Studies in mice transgenic for the HLA alleleDRB1*0401showed that conversion of arginine to citrulline in the position interacting with SE in-creased peptide-MHC binding affinity and led to the efficient activation of CD4+T cells in these mice.40
Two recent studies compared several SE genotypes in a cohort of RA patients with anti-CCP2 (see below for anti-CCP2 test) and showed that the presence of one SE allele appeared to be strongly associated with the pro-duction of anti-CCP2.41,42Moreover, patients who were homozygous for SE
alleles. These observations, combined with previous findings that SE alleles are highly correlated with production of ACPA in RA patients, strongly sup-port the hypothesis that these HLA SE-containing molecules play a role in the activation of CD4+T cells through preferential presentation of citrulli-nated antigens. These “citrulline”-specific T cells may then trigger the IgG antibody response to citrullinated antigens.
2. PADI4 Gene SNPs Associated with RA
All genes coding isotypes of PAD are located in a single cluster. The human PAD gene cluster spans a 350 kb segment in chromosome 1p36.1.
The PADI4 gene has two major haplotypes, one of which has been shown
to be a susceptibility locus for RA. The two haplotypes consist of four SNPs (163G-A, 245T-C, 335G-C, and 349T-G). These SNPs are thought to make
PADI4 mRNA more resistant to degradation, suggesting that protein levels of PAD4 might be elevated in patients with this haplotype. Gene linkage studies of the various genes encoding PAD have revealed that a certain hap-lotype of thePADI4gene, the gene encoding PAD4, was associated with RA in a Japanese population.43In this study, anti-CCP antibodies were found in 87% of RA patients homozygous for the susceptible haplotype, compared to 67% of heterozygous and non-RA haplotype carrying RA patients. The relative risk of RA in individuals with two copies of the susceptible haplo-type is 1.97 compared with individuals without a copy of the susceptible haplotype.43
These data however should be interpreted with caution because an asso-ciation of thisPADI4haplotype with RA could not be confirmed in a cohort of RA patients in the United Kingdom,44nor in a French population.45
Fur-thermore, nothing is known about the relationship between these amino acid replacements and protein function. Thus, future studies are necessary to validate the relevance of certainPADI4haplotypes in RA.
3. Smoking and RA
IV. ACPA IN THE PATHOGENESIS OF RA
Circumstantial evidence suggests an intimate relationship between im-munity against citrullinated antigens and RA. Considering that antibodies against these antigens can persist for years without apparent arthritis, it is likely that additional environmental and genetic factors have to come to-gether within one individual for the clinical expression of the disease.
Citrullination has been described as a physiological process during apop-tosis at multiple sites in the body but it can also occur during inflammation. Despite the fact that inflammation is a common event in everyone’s daily life, <1% of the population develops ACPA. Therefore, it is conceivable that an accumulation of genetic and environmental factors is necessary for a pathologic response to citrullinated antigens to develop. It is possible that citrullination of proteins as a result of inflammation will initiate an HLA class II-restricted T-cell response only in a genetically predisposed (e.g., SE-positive) individual.39
As previously discussed, PAD are normally present in an inactive state, as they require high concentrations of calcium for activation. During cell death, when the integrity of the cell membrane is disrupted, PAD may leak out of the cell and become activated, leading to citrullination of the extracellular ma-trix proteins and thereby generating the target antigens for ACPA. PAD4 and PAD2 are expressed by granulocytes, monocytes, and macrophages. Recruit-ment of granulocytes and monocytes to an inflamed joint will most probably result in the activation of these two enzymes, allowing the citrullination of intra- and extracellular proteins, including extracellular fibrin.
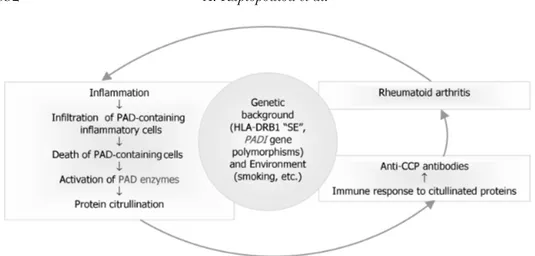
Recognition of citrullinated proteins by ACPA, already present in the systemic circulation, leads to formation of immune complexes, complement activation, and further attraction of granulocytes, monocytes, macrophages, and mast cells. As a result, inflammation seems to cause even more anti-gen to be produced, leading the immune response in a vicious circle that perpetuates the inflammatory process (Figure 3).
V. ANTI-CCP: ANTI-CYCLIC CITRULLINATED PEPTIDE ANTIBODIES
FIGURE 3 Probable role of anti-CCP antibodies in the pathogenesis of RA. Citrullination can occur dur-ing inflammation. Recruitment of granulocytes and monocytes to the inflamed joint may result in leakage and subsequent activation of PAD, allowing the citrullination of intra- and extracellular proteins. Citrul-lination of proteins will initiate an HLA class II-restricted T-cell response preferentially in a genetically predisposed (e.g., SE-positive) individual. Recognition of citrullinated proteins by ACPA, already present in the systemic circulation, leads to immune complex formation, complement activation, and further attraction of inflammatory cells and antigen production, resulting in perpetuation of the inflammatory process. Additional environmental (e.g., smoking) and genetic factors (PAD polymorphisms) have to come together for the clinical expression of the disease.
Several reports have described the diagnostic performance of assays de-fecting antibodies to CPP (anti-CCP).48−51Such autoantibodies are very
spe-cific for RA (up to 98%), but they are also detected very early in the disease or even several years before disease onset.52 Their titer tends to correlate
with the evolvement of erosive RA.
A. Anti-CCP1 and Anti-CCP2
1. Anti-CCP1
The first commercially available ELISA (CCP1, Immunoscan RA, Euro-diagnostica) was developed using a cyclic citrullinated synthetic peptide de-rived from the sequence of human filaggrin as immunosorbent.53
2. Anti-CCP2
In an attempt to increase the RA sera positive percentage in an anti-CCP assay, a library of citrullinated peptides was screened with a pool of RA sera, and a number of highly reactive peptides were identified. Those peptides are currently used in the second generation test. This anti-CCP2 test has increased both sensitivity and specificity for RA (about 82% and 98–99% respectively).52,54 Several different commercial kits are now available, and,
TABLE 2 Diagnostic Sensitivity of Various Tests for the Detection of ACPA compared to RF#
Test Diagnostic Sensitivity(%)∗
AFA 38
AKA 43–52
Anti-Sa 43
Anti-CCP 58
RF 16
#Adapted from Vincent 2005.58
∗Diagnostic sensitivity at a diagnostic specificity of 98.5%, in
a cohort of 711 patients with established RA or other rheumatic diseases.
B. Anti-CCP Versus RF
Recent studies confirm that the anti-CCP2 test has a sensitivity that is com-parable to that of RF (80%) but combines this with far better specificity.55,56 The fact that about 40% of RF-seronegative patients are anti-CCP2-positive underscores the additional diagnostic potential of anti-CCP2 used in combi-nation with RF (Tables 2 & 3).57,58
C. CCP in Distinguishing RA from Other Diseases
Numerous studies show that the anti-CCP test may enable clinicians to effectively distinguish RA patients from those with similar diseases, even in cases where the RF is not discriminatory (Table 4).59
1. Hepatitis C Virus (HCV)
A significant proportion of patients with chronic HCV infection suffers from a symmetric inflammatory polyarthritis that sometimes closely resem-bles the symptoms of RA. RF cannot be used to discriminate HCV-associated arthritis from RA as the majority of these patients are RF positive.60,61 The
anti-CCP2 test is a helpful diagnostic tool in this group of patients, as anti-CCP positivity is negligible compared to that in RA patients.62
2. Systemic Lupus Erythematosus (SLE)
Differential diagnosis of SLE from RA can sometimes be difficult, espe-cially when it is associated with polyarthritis at presentation Furthermore,
TABLE 3 Value of Anti-CCP and RF for RA Diagnosis∗
Anti-CCP1 Anti-CCP2 RF
Sensitivity (%) 54 68.5 65
Specificity (%) 97 97 81
TABLE 4 Approximate Prevalence of Anti-CCP in Rheumatic Diseases Other Than RA59
Disease Prevalence of anti-CCP2 (%)
SLE 9
SS 5
HCV 1
Wegener granulomatosis 1
Ankylosing spondylitis 3
Psoriatic arthritis 8
Polymyalgia rheumatica 0
although uncommon, both diseases can co-exist in the same patient, the so-called Rupus. The anti-CCP2 test seems to be a powerful diagnostic tool, as cumulative data indicate that anti-CCP2 test positivity reaches a low per-centage of approximately 9% in SLE patients.59Additionally, in a large SLE patient cohort, 10 of whom had erosive joint disease, only two were anti-CCP1 positive; in contrast, only 0.5% of the non-erosive SLE patients were anti-CCP1 positive, versus 18% for RF.63
3. Sjogren Syndrome (SS)
SS is a chronic autoimmune exocrinopathy disorder characteristically affecting the salivary and lacrimal glands (primary SS). Secondary SS appears as a feature of other systemic diseases, such as RA. RF is positive in 40– 70% of patients with primary SS. Several studies showed that anti-CCP is present in approximately 5% of primary SS patients, in contrast to the high prevalence of RF in patients with primary SS. Anti-CCP-positive patients with SS may, however, be prone to develop RA and thus require close clinical and radiographic follow-up.64,65
4. Seronegative (RF-Negative) Arthritides
The seronegative arthritides (or spondyloarthropathies) constitute a group of arthritides that includes reactive arthritis, psoriatic arthritis, and ankylosing spondylitis. These diseases are characterized by involvement of both the axial skeleton (spine, shoulders, sacroiliac joints) and peripheral joints, with inflammation at the site of the entheses (enthesopathy). Anti-CCP positivity was also compared in psoriatic arthritis66 and in ankylosing spondylitis, with a mean prevalence of 8% and 3% respectively.59
5. Juvenile Idiopathic Arthritis (JIA)
Data on anti-CCP positivity in JIA are conflicting; this may be a reflection of the heterogeneity of the disease.67−71Avcinet al.67showed that in 109 JIA patients, anti-CCP were detected in only two RF-negative patients (2%). van Rossumet al.,68 in a small cohort of JIA patients, showed that anti-CCP are present (73%) in only a subset of polyarticular IgM RF-positive JIA patients. In contrast, Low et al.,69 in a total of 66 JIA patients, detected significant concentrations of anti-CCP in the majority (77%) of RF-positive polyarthritis patients. Ferucciet al.70followed 230 JIA patients and demonstrated that, al-though the overall prevalence of anti-CCP in JIA was low (5.6%), a substantial proportion of RF-positive patients with polyarthritis were anti-CCP positive. Finally, Kwoket al.,71in 59 Chinese patients with JIA, showed that anti-CCP had high specificity (99.1%) but low sensitivity (10.2%) for this disease. In conclusion, the low sensitivity of anti-CCP in JIA does not allow its use as a screening test. However, because of its high specificity, it may become one of the most useful predictive serological tests for joint erosions in JIA of the polyarticular RF-positive subset.
6. Other Inflammatory Conditions
Makrygiannakis et al.72 recently showed that citrullinated proteins are present in a wide range of inflammatory tissues, which suggests that this pro-cess is inflammation- rather than disease-dependent. These findings, how-ever, require further verification.
D. Anti-CCP Test: Prognostic Value
With the availability of more sophisticated and effective therapies and with the understanding that early intervention is crucial in preventing irre-versible joint damage, it is widely accepted that early and accurate diagnosis of RA is critical in disease management.73,74 To facilitate early diagnosis, a serological marker of RA that is detectable very early in the disease is needed. As already mentioned, several recent studies have shown that anti-CCP can be detected very early in the course of RA and may be helpful in early diagnosis (Table 5).
TABLE 5 Predictive Value of Anti-CCP2 Measurement for RA Diagnosis#
Early undifferentiated
arthritis Healthy
OR for RA Diagnosis
(95% confidence interval) 25 (18–35) 28 (8–95)∗/15.9∗∗
#Adapted from Avouac 2006.59
∗Rantapaa-Dahlqvist 2003.51
1. Blood Donor Cohorts
Studies using pre-disease serum samples from RA patients that were for-mer blood donors have shown that both RF and anti-CCP can be detected years before manifestation of the first symptoms of the disease. Rantapaa-Dahlqvistet al.51investigated two different Swedish cohorts and identified 83 future RA patients. In samples examined nine years and more than 1.5 years prior to symptom onset, anti-CCP predicted the development of the disease with a sensitivity of 4% and 25%, respectively, and a specificity of 98%. In sam-ples examined within 1.5 years of the disease onset, the sensitivity increased to 52%, and the specificity remained high (98%), while the sensitivity of IgM RF was 30%.
In a subanalysis of the same cohort, Berglinet al.75analyzed the presence of SE and anti-CCP2. Two years prior to symptom onset, the sensitivity and specificity of anti-CCP2 and SE as predictors of future development of RA were 37% and 98%, respectively. However, in a multivariate analysis with logistic regression test, anti-CCP2 had the highest predictive value, with an odds ratio (OR) of 15.9 (versus 6.8 and 2.35 for IgA RF and SE, respectively). Finally, Nielen et al.76 investigated 79 patients; five years before symptom
onset, the sensitivity and specificity of anti-CCP2 to predict the occurrence of RA were 29% and 99.5%, respectively.59Together, these studies indicate that the presence of anti-CCP (and RF) can be detected early in the development of RA and that their presence can be predictive for the development of the disease. (Table 5)
2. Early Undifferentiated Arthritis
A significant percentage of patients (up to 40%) do not fulfill criteria for any of the known arthritides within the first 3–12 months of the disease. Predicting the patients who are likely to develop persistent RA-like disease with erosions is of paramount importance in establishing prognosis and ap-propriate treatment.
Among 11 studies performed on early arthritis prediction, the mean symptom duration at baseline was<9.5±10 months. Those studies included a total of 2877 patients with a mean follow-up of 17±8 months. At the end of the follow-up, 51% of patients were classified as having RA. Of patients with early arthritis at baseline, 23 ±5% had positive anti-CCP2, and 23± 6% had positive anti-CCP1. Of patients classified as having RA at the time of the diagnosis, 51±8% had positive anti-CCP2, and 46±6% had positive anti-CCP1. Thus, the mean OR (which characterizes the risk to develop RA in those patients with undifferentiated arthritis) was 25 for anti-CCP2 and 20 for anti-CCP1.59
van Gaalenet al.78using the anti-CCP2 test, 318 out of 936 patients attending an early arthritis clinic could not be diagnosed as having RA and thus were classified as having undifferentiated arthritis. After three years of follow-up, 40% of these patients were clinically classified as RA patients. Of the patients that were negative for anti-CCP2 at baseline, 25% developed RA in three years. By contrast, of the patients with a positive anti-CCP2 test at baseline, 93% developed RA. These findings were recently confirmed by a study of 314 early arthritis patients by Vittecoqet al.79; after a follow-up at one year, 90% of patients that were anti-CCP2 positive at baseline were classified as having established RA (Table 5).
3. Prediction of Erosive Arthritis
Several studies have reported the prognostic ability of the CCP1 test to predict erosive progression of RA. After six years of follow-up of RA patients, Kroot et al.54 reported that anti-CCP1-positive patients developed
signifi-cantly more severe radiological damage than anti-CCP1-negative patients. Meyer et al.57 followed 191 patients with recent onset RA and concluded
that the likelihood of increased erosive damage after five years was signifi-cantly greater in the anti-CCP1-positive group (67%; OR 2.5) compared to the RF-positive group (OR 0.7).
Several other reports also show that anti-CCP positivity in early arthritis patients at baseline is a strong prognostic marker for the development of ero-sive RA in the near future. Vencovsky et al.80 suggested that combining the potential of the anti-CCP test with the RF test provides the highest prognostic value for the prediction of erosive and progressive RA. Visser et al.81 devel-oped a prediction model to discriminate between self-limiting, non-erosive, and persistent erosive arthritis and applied them to a cohort of 524 early arthritis patients. The superiority of their model over the standard American College of Rheumatology criteria in predicting persistent versus self-limiting disease and non-erosive versus erosive persistent disease was largely depen-dent on the anti-CCP1 parameter. Recent studies using the anti-CCP2 test also demonstrated the prognostic value of anti-CCP for RA development.56,82 Forslindet al.83 assessed anti-CCP2 at baseline in 379 patients and mea-sured radiological joint damage and progression after two years of follow-up. They concluded that the presence of anti-CCP2 was associated with signifi-cantly higher levels of erosion compared to RF and other parameters. Using a similar approach, Kastbom et al.84 showed that anti-CCP2 had similar di-agnostic sensitivity to RF in early RA but was superior as a predictor of the disease course over three years. Interestingly, anti-CCP positivity remained essentially unchanged three years after diagnosis.
E. Effect of Treatment on Anti-CCP Production
One of the important questions in the management of autoimmune diseases is whether the effect of treatment can be monitored by the level of autoantibodies measured.
1. DMARDS
Several studies conclude that, although serum levels of anti-CCP do not seem to correlate with disease activity, they often drop after institution of DMARDS, at least in RA of short duration.85−87
2. Anti-TNFα
Inhibitors of the proinflammatory cytokine TNFαhave been found to be effective in the treatment of RA. Correlation of anti-CCP levels with response to anti-TNFαtreatment (Infliximab) has not been convincingly sustained in several studies conducted so far in relatively small cohorts of patients.88In
a cohort of 90 RA patients,89another broadly used anti-TNFα(Etanercept), when combined with DMARDS in the treatment of RA, led to a decrease in anti-CCP and RF serum levels, which paralleled a reduction in clinical disease activity.
VI. CONCLUSIONS
RA patients produce a variety of autoantibodies, many of which are not specific to the disease. Autoantibodies to citrullinated proteins (APF, AKA, anti-filaggrin, anti-CCP, and anti-Sa), however, have a much higher specificity for RA. Of these, anti-CCP are of special interest because of their high sensi-tivity and specificity for RA and their ability to predict erosive arthritis. These autoantibodies may serve as a powerful serologic marker for early diagnosis and prognosis for RA. Routine testing of these RA-specific autoantibodies may prove to be useful in establishing new criteria for early RA diagnosis and classification. Since these antibodies can persist for years without ap-parent arthritis, it is likely that environmental and genetic factors have to come together within one individual for the induction and progression of RA.
The presence of anti-CCP in healthy individuals and in patients with early and undifferentiated arthritis predicts with high probability (approx-imately 30%, Table 5) that they will develop RA. Identification of patients with aggressive and erosive arthritis prior to the occurrence of joint dam-age may lead to targeted use of newly developed drugs in those patients. This would potentially break the inflammatory process in RA joints. Finally, these autoantibodies, together with RF and probably other surrogate genetic or biochemical markers, may facilitate primary prevention of RA by allowing high-risk persons to be treated with agents such as hydroxychloroquine prior to the onset of the disease.
REFERENCES
[1] Vossenaar ER, van Venrooij WJ. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis.Arthritis Res Ther2004;6: 107–111.
[2] Yamada R, Suzuki A, Chang X, Yamamoto K. Citrullinated proteins in rheumatoid arthritis.Front Biosci2005;10: 54–64.
[3] Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L. Almost all about citrulline in mammals.Amino Acids2005;29: 177–205.
[4] Nijenhuis S, Zendman AJ, Vossenaar ER, Pruijn GJ, vanVenrooij WJ. Autoantibodies to citrullinated proteins in rheumatoid arthritis: clinical performance and biochemical aspects of a RA-specific marker.Clin Chim Acta2004;350: 17–34.
[5] Watanabe K, Senshu T. Isolation and characterization of cDNA clones encoding rat skeletal muscle peptidylarginine deiminase.J Biol Chem1989;264: 15255–15260.
[6] Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease.Bioessays2003;25: 1106–1118.
[7] Vossenaar ER, Radstake TR, van der Heijden A, van Mansum MA, Dieteren C, de Rooij DJ, Barrera P, Zendman AJ, van Venrooij WJ. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis2004;63: 373–381.
[8] Rogers G, Winter B, McLaughlan C, Powell B, Nesci T. Peptidylarginine deiminase of the hair follicle: characterization, localization, and function in keratinizing tissues.J Invest Dermatol1997; 108: 700–707.
[9] Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature.J Clin Invest1994;94: 146–154.
[10] Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes.J Biol Chem2002;277: 49562–49568.
[11] Nakashima K, Hagiwara T, Ishigami A, Nagata S, Asaga H, Kuramoto M, Senshu T, Yamada M. Molecular characterization of peptidylarginine deiminase in HL-60 cells induced by retinoic acid and 1alpha,25-dihydroxyvitamin D(3).J Biol Chem1999;274: 27786–27792.
[12] Yamakoshi A, Ono H, Nishijyo T, Shiraiwa M, Takahara H. Cloning of cDNA encoding a novel isoform (type IV) of peptidylarginine deiminase from rat epidermis.Biochim Biophys Acta1998; 1386: 227–232.
[13] Ishigami A, Kuramoto M, Yamada M, Watanabe K, Senshu T. Molecular cloning of two novel types of peptidylarginine deiminase cDNAs from retinoic acid-treated culture of a newborn rat keratinocyte cell line.FEBS Lett1998;433: 113–118.
[14] Hingorani K, Szebeni A, Olson MO. Mapping the functional domains of nucleolar protein B23.
J Biol Chem2000;275: 24451–24457.
[15] Okuda M. The role of nucleophosmin in centrosome duplication. Oncogene2002;21: 6170–6174. [16] Takemura M, Ohoka F, Perpelescu M, Ogawa M, Matsushita H, Takaba T, Akiyama T, Umekawa
[17] Chavanas S, Mechin MC, Takahara H, Kawada A, Nachat R, Serre G, Simon M. Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6.Gene2004;330: 19–27.
[18] Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin.J Biol Chem1996;271: 30709–30716.
[19] van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value.Arthritis Res2002;4: 87–93. [20] Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of
arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule.J Immunol2003;171: 538–541.
[21] van Gaalen F, Ioan-Facsinay A, Huizinga TW, Toes RE. The devil in the details: the emerging role of anticitrulline autoimmunity in rheumatoid arthritis.J Immunol2005;175: 5575–5580.
[22] Senshu T, Kan S, Ogawa H, Manabe M Asaga H. Preferential deimination of keratin K1 and filaggrin during the terminal differentiation of human epidermis.Biochem Biophys Res Commun1996;225: 712–719.
[23] Chang X, Han J, Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors.Mol Carcinog2006;45: 183–196.
[24] Chang X, Yamada R, Sawada T, Suzuki A, Kochi O, Yamamoto K. Citrullination of fibronectin in synovial tissue of rheumatoid arthritis.Rheumatology (Oxford)2005;44: 1374–1382.
[25] Weyand CM, Goronzy JJ. Pathomechanisms in rheumatoid arthritis—time for a string theory?J Clin Invest2006;116: 869–871.
[26] Nienhuis RL, Mandema E. A new serum factor in patients with rheumatoid arthritis; the antiper-inuclear factor.Ann Rheum Dis1964;23: 302–305.
[27] Hoet RM, Boerbooms AM, Arends M, Ruiter DJ, van Venrooij WJ. Antiperinuclear factor, a marker autoantibody for rheumatoid arthritis: colocalisation of the perinuclear factor and profilaggrin.
Ann Rheum Dis1991;50: 611–618.
[28] Young BJ, Mallya RK, Leslie RD, Clark CJ, Hamblin TJ. Anti-keratin antibodies in rheumatoid arthritis.Br Med J1979;2: 97–99.
[29] Sebbag M, Simon M, Vincent C, Masson-Bessiere C, Girbal E, Durieux JJ, Serre G. The antiper-inuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies.J Clin Invest1995;95: 2672–2679.
[30] Despres N, Boire G, Lopez-Longo FJ, Menard HA. The Sa system: a novel antigen–antibody system specific for rheumatoid arthritis.J Rheumatol1994;21: 1027–1033.
[31] Hayem G, Chazerain P, Combe B, Elias A, Haim T, Nicaise P, Benali K, Eliaou JF, Kahn MF, Sany J, Meyer O. Anti-Sa antibody is an accurate diagnostic and prognostic marker in adult rheumatoid arthritis.J Rheumatol1999;26: 7–13.
[32] Hueber W, Hassfeld W, Smolen JS, Steiner G. Sensitivity and specificity of anti-Sa autoantibodies for rheumatoid arthritis.Rheumatology (Oxford)1999;38: 155–159.
[33] Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies.J Clin Invest1998;101: 273–281.
[34] Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Noqueira L, Vincent C, Senshu T, Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin.J Immunol2001;166: 4177–4184. [35] Vossenaar ER, Despres N, Lapointe E, van der Heijden A, Lora M, Senshu T, van Venrooij WJ,
Menard HA. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin.Arthritis Res Ther2004;6: R142–R150.
[36] Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase v and histone deimination in granulocytes.J Biol Chem2002;277: 49562–49568.
[37] Chapuy-Regaud S, Sebbag M, Nachat R, Baeten D, Foulquier V, Simon M, Senshue T, Yamada M, Takahara H, De Keyser F, Serre G. Peptidylarginine deiminase isoforms expressed in the synovial membrane of rheumatoid arthritis patients.Arthritis Res Ther2003;5(Suppl 1): 5 (abstract). [38] Linn-Rasker SP, van der Helm-van Mil AH, van Gaalen FA, Kloppenburg M, de Vries RR, le Cessie
[39] Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, Ronnelid J, Harris HE, Ulfgren AK, Rantapaa-Dahlqvist S, Eklund A, Padyukov L, Alfredsson L. A new model for an eti-ology of rheumatoid arthritis: smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination.Arthritis Rheum2006;54: 38–46.
[40] Rubin B, Sonderstrup G. Citrullination of self-proteins and autoimmunity.Scand J Immunol2004; 60: 112–120.
[41] van Gaalen FA, van Aken J, Huizinga TW, Schreuder GM, Breedveld FC, Zanelli E, van Venrooij WJ, Verweij CL, Toes RE, de Vries RR. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) affects severity of rheumatoid arthritis.Arthritis Rheum2004; 50: 2113–2121.
[42] Berglin E, Padyukov L, Sundin U, Hallmans G, Stenlund H, van Venrooij WJ, Klareskog L, Dahlqvist SR. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis.Arthritis Res Ther2004;6: R303–R308.
[43] Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M, Nagasaki M, Nakayama-Hamada M, Kawaida R, Ono M, Ohtsuki M, Furukawa H, Yoshino S, Yukioka M, Tohma S, Matsubara T, Wakitani S, Teshima R, Nishioka Y, Sekine A, Iida A, Takahashi A, Tsunoda T, Nakamura Y, Yamamoto K. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis.Nat Genet2003;34: 395–402.
[44] Barton A, Bowes J, Eyre S, Spreckley K, Hinks A, John S, Worthington J. A functional haplotype of the PADI4 gene associated with rheumatoid arthritis in a Japanese population is not associated in a United Kingdom population.Arthritis Rheum2004;50: 1117–1121.
[45] Caponi L, Petit-Teixeira E, Sebbag M, Bongiorni F, Moscato S, Pratesi F, Pierlot C, Osorio J, Chapuy-Regaud S, Guerrin M, Cornelis F, Serre G, Migliorini P; ECRAF. A family based study shows no association between rheumatoid arthritis and the PADI4 gene in a white French population.Ann Rheum Dis2005;64: 587–593.
[46] Mimori T. Clinical significance of anti-CCP antibodies in rheumatoid arthritis.Intern Med2005;44: 1122–1126.
[47] Schellekens GA, Visser H,.de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide.Arthritis Rheum2000;43: 155–163.
[48] Goldbach-Mansky R, Lee J, McCoy A, Hoxworth J, Yarboro C, Smolen JS, Steiner G, Rosen A, Zhang C, Menard HA, Zhou ZJ, Palosuo T, Van Venrooij WJ, Wilder RL, Klippel JH, Schumacher HR Jr, El-Gabalawy HS. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset.Arthritis Res2000;2: 236–243.
[49] Bizzaro N, Mazzanti G, Tonutti E, Villalta D, Tozzoli R. Diagnostic accuracy of the anti-citrulline antibody assay for rheumatoid arthritis.Clin Chem2001;47: 1089–1093.
[50] Bas S, Perneger TV, Seitz M, Tiercy JM, Roux-Lombard P, Guerne PA. Diagnostic tests for rheuma-toid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors.Rheumatology (Oxford)2002;41: 809–814.
[51] Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis.Arthritis Rheum2003;48: 2741–2749.
[52] Vossenaar ER, van Venrooij WJ. Anti-CCP antibodies, a highly specific marker for (early) rheuma-toid arthritis.Clin Appl Immunol Rev2004;4: 239–262.
[53] Jansen AL, van der Horst-Bruinsma I, van Schaardenburg D, van de Stadt RJ, de Koning MH, Dijkmans BA. Rheumatoid factor and antibodies to cyclic citrullinated peptide differentiate rheumatoid arthritis from undifferentiated polyarthritis in patients with early arthritis.J Rheumatol
2002;29: 2074–2076.
[54] Kroot EJ, de Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, van’t Hof M, van de Putte LB, van Rijswijk MH, van Venrooij WJ, van Riel PL. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis.Arthritis Rheum2000;43: 1831– 1835.
[56] De Rycke L, Peene I, Hoffman IE, Kruithof E, Union A, Meheus L, Lebeer K, Wyns B, Vincent C, Mielants H, Boullart L, Serre G, Veys EM, De Keyser F. Rheumatoid factor and anti-citrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progres-sion rate, and extraarticular manifestations.Ann Rheum Dis2004;63: 1587–1593.
[57] Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A, Nicaise-Roland P, Sibilia J, Combe B. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage.Ann Rheum Dis2003;62: 120–126.
[58] Vincent C, Nogueira L, Clavel C, Sebbag M, Serre G. Autoantibodies to citrullinated proteins: ACPA.Autoimmunity2005;38: 17–24.
[59] Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review.Ann Rheum Dis2006;65: 845–851. [60] Palazzi C, Padula A. Hepatitis C virus and arthritis.Rheum Dis Clin North Am 2003; 29: 111–
122.
[61] Wener MH, Hutchinson K, Morishima C, Gretch DR. Absence of antibodies to cyclic citrullinated peptide in sera of patients with hepatitis C virus infection and cryoglobulinemia.Arthritis Rheum
2004;50: 2305–2308.
[62] Bombardieri M, Alessandri C, Labbadia G, Iannuccelli C, Carlucci F, Riccieri V, Paoletti V, Valesini G. Role of anti-cyclic citrullinated peptide antibodies in discriminating patients with rheuma-toid arthritis from patients with chronic hepatitis C infection-associated polyarticular involvement.
Arthritis Res Ther2004;6: R137–R141.
[63] Mediwake R, Isenberg DA, Schellekens GA, van Venrooij WJ. Use of anti-citrullinated peptide and anti-RA33 antibodies in distinguishing erosive arthritis in patients with systemic lupus erythemato-sus and rheumatoid arthritis.Ann Rheum Dis2001;60: 67–68.
[64] van Noord C, Hooijkaas H, Dufour-van den Goorbergh BC, van Hagen PM, van Daele PL, van de Merwe JP. Diagnostic value of anti-cyclic citrullinated peptide antibodies to detect rheumatoid arthritis in patients with Sj¨ogren’s syndrome.Ann Rheum Dis2005;64: 160–162.
[65] Gottenberg JE, Mignot S, Nicaise-Rolland P, Cohen-Solal J, Aucouturier F, Goetz J, Labarre C, Meyer O, Sibilia J, Mariette X. Prevalence of anti-cyclic citrullinated peptide and anti-keratin antibodies in patients with primary Sjogren’s syndrome.Ann Rheum Dis2005;64: 114–117.
[66] Alenius GM, Berglin E, Rantap¨a¨a Dahlqvist S. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation.Ann Rheum Dis2006;65: 398–400. [67] Avcin T, Cimaz R, Falcini F, Zulian F, Martini G, Simonini G, Porenta-Besic V, Cecchini G, Borghi
MO, Meroni PL. Prevalence and clinical significance of anti-cyclic citrullinated peptide antibodies in juvenile idiopathic arthritis.Ann Rheum Dis2002;61: 608–611.
[68] van Rossum M, van Soebergen R, de Kort S, ten Cate R, Zwinderman AH, de Jong B, Dijkmans B, van Venrooij WJ. Anti-cyclic citrullinated peptide (anti-CCP) antibodies in children with juvenile idiopathic arthritis.J Rheumatol2003;30: 825–828.
[69] Low JM, Chauhan AK, Kietz DA, Daud U, Pepmueller PH, Moore TL. Determination of anti-cyclic citrullinated peptide antibodies in the sera of patients with juvenile idiopathic arthritis.J Rheumatol
2004;31: 1829–1833.
[70] Ferucci ED, Majka DS, Parrish LA, Moroldo MB, Ryan M, Passo M, Thompson SD, Deane KD, Rewers M, Arend WP, Glass DN, Norris JM, Holers VM. Antibodies against cyclic citrullinated peptide are associated with HLA-DR 4 in simplex and multiplex polyarticular-onset juvenile rheumatoid arthritis.Arthritis Rheum2005;52: 239–246.
[71] Kwok JS, Hui KH, Lee TL, Wong W, Lau YL, Wong RW, Kim DL, Jones BM. Anti-cyclic citrullinated peptide: diagnostic and prognostic values in juvenile idiopathic arthritis and rheumatoid arthritis in a Chinese population.Scand J Rheumatol2005;34: 359–366.
[72] Makrygiannakis D, af Klint E, Lundberg IE, Loftberg R, Ulfgren AK, Klareskog L, Catrina AI. Citrullination is an inflammation dependent processAnn Rheum Dis2006;65: 1219–1222. [73] O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity?Arthritis Rheum2002;46:
283–285.
[74] Landewe RB, Boers M, Verhoeven AC, Westhovens R, van de Laar MA, Markusse HM, van Denderen JC, Westedt ML, Peeters AJ, Dijkmans BA, Jacobs P, Boonen A, van der Heijde DM, van der Linden S. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention.Arthritis Rheum2002;46: 347–356.
antigens is strongly associated with future onset of rheumatoid arthritis.Arthritis Res Ther2004;6: R303–R308.
[76] Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors.Arthritis Rheum
2004;50: 380–386.
[77] Jansen AL, van der Horst-Bruinsma IE, van Schaardenburg D, van de Stadt RJ, de Koning MH, Dijkmans BA. Rheumatoid factor and antibodies to cyclic citrullinated peptide differentiate rheumatoid arthritis from undifferentiated polyarthritis in patients with early arthritis.J Rheumatol
2002;29: 2074–2076.
[78] van Gaalen FA, Linn-Rasker S, van Venrooij WJ, de Jong BA, Breedveld FC, Verweij CL, Toes RE, Huizinga TW. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis.Arthritis Rheum2004;50: 709–715.
[79] Vittecoq O, Incaurgarat B, Jouen-Beades F, Legoedec J, Letourneur O, Rolland D, Gervasi G, Menard JF, Gayet A, Fardellone P, Daragon A, Jolivet M, le Loet X, Tron F, Autoantibodies recog-nizing citrullinated rat filaggrin in an ELISA using citrullinated and non-citrullinated recombinant proteins as antigens are highly diagnostic for rheumatoid arthritis.Clin Exp Immunol2004;135: 173– 180.
[80] Vencovsky J, Machacek S, Sedova L, Kafkova J, Gatterova J, Pesakova V, Ruzickova S. Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis.Ann Rheum Dis2003; 62: 427–430.
[81] Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis.Arthritis Rheum2002;46: 357–365.
[82] Jansen LM, van Schaardenburg D, van der Horst-Bruinsma IE, van der Stadt RJ, de Koning MH, Dijkmans BA. The predictive value of anti-cyclic citrullinated peptide antibodies in early arthritis.
J Rheumatol2003;30: 1691–1695.
[83] Forslind K, Ahlmen M, Eberhardt K, Hafstrom I, Svensson B; BARFOT Study Group. Prediction of radiological outcome in early RA in clinical practice: role of antibodies to citrullinated peptides (anti-CCP).Ann Rheum Dis2004;63: 1090–1095.
[84] Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti-CCP antibody test predicts the disease course during three years in early rheumatoid arthritis (the TIRA project).Ann Rheum Dis2004;63: 1085– 1089.
[85] van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value.Arthritis Res2002;4: 87–93. [86] Marcelletti JF, Nakamura RM. Assessment of serological markers associated with rheumatoid
arthritis: diagnostic autoantibodies and conventional disease activity markers.Clin Appl Immunol Rev2003;4: 109–123.
[87] Steiner G, Smolen J. Autoantibodies in rheumatoid arthritis and their clinical significance.Arthritis Res2002;4(Suppl 2): S1–S5.
[88] Bobbio-Pallavicini F, Alpini C, Caporali R, Avalle S, Bugatti S, Montecucco C. Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment.Arthritis Res Ther2004;6: R264– R272.