Huddling up in a dry environment: the physiological benefits of aggregation in an intertidal gastropod
Texto completo
(2) 1120. during tidal emersion periods. More specifically, acknowledging the functions of several factors that modulate the expression of heat-shock protein genes, involved in ameliorating the effects of overheating, has allowed researchers to suggest that many intertidal organisms are living at or close to their thermal limits (Hofman 1999; Helmuth et al. 2006; Somero 2002; Tomanek 2010; Dong et al. 2011; Marshall and McQuad 2011). Among the aforementioned responses to thermal stress, behavioral adjustments are mentioned recurrently in the literature, particularly social behaviors such as aggregation of individuals or individual isolation from surroundings and conspecifics. Although these behaviors have often been assumed to be associated with water conservation and the regulation of body temperature, a consensus is still lacking on the purpose behind these behavioral adjustments or the mechanisms linking the physiological responses to specific behaviors as described above (Garrity 1984; Chapman and Underwood 1996; Muñoz et al. 2005; Chapperon and Seuront 2011a, b; Stafford et al. 2012). Thus, one of the current challenges is to improve our understanding of the physiological and behavioral capabilities of organisms to adapt to changing physical conditions if we are to predict how organisms will adapt to larger climatic changes (Helmuth et al. 2005; Bozinovic et al. 2011). Intertidal gastropods, a largely mobile group of marine invertebrates, exhibit certain physiological, morphological and, in particular, behavioral traits which allow them to cope with thermal stress and desiccation risk during emersion (Garrity 1984; Somero 2002; Helmuth et al. 2006; Lee and Lim 2009; Chapperon and Seuront 2011a, b; Miller and Denny 2011). One common behavior is the closing of the opercular opening using a lid or ‘‘trapdoor’’ attached to the muscular foot, enclosing the soft body tissues within the calcareous shell. When the operculum is closed, gastropods are also anchored to the substrata hermetically with a mucus seal, effectively reducing water loss by evaporation, cutting off gas exchange with the surrounding environment (Gendron 1977; Garrity 1984; Hughes 1986; Bates et al. 2005; Denny et al. 2006). A second behavior commonly observed is the formation of dense aggregations of individuals near rock crevices or scars. It is generally thought that by joining an aggregation, individuals experience reduced thermal stress by collectively forming a wet microclimate, allowing for greater water conservation and maintenance of cooler body temperature when exposed to warm terrestrial conditions (Hughes 1986; Chapman 1995; Chapman and Underwood 1996; Rojas et al. 2000; Chapperon and Seuront 2011a, b). Although field studies have examined the relationship between thermal stress amelioration and snail aggregations, conclusive results suggesting that microclimatic effects could promote aggregation behavior are still lacking. 123. Mar Biol (2013) 160:1119–1126. (Chapman 1995; Chapman and Underwood 1996; Rojas et al. 2000; Stafford et al. 2012) or even convey an antipredator defense (Coleman et al. 1999; Coleman 2010). Identifying the physiological tradeoffs that occur during tidal emersion is critical in resolving the relationship between aggregation behavior and the amelioration of thermal stress. With the operculum closed, isolated snails (nonaggregated individuals) may limit their water loss due to desiccation, but as a consequence may also reduce their oxygen supply due to limited gas exchange. This, in turn, would result in a loss of efficiency in the physiological machinery of the heat-shock protein system that is critical in combating thermal stress (Dahlhoff et al. 2001; Somero 2002; Tomanek 2002; Dong et al. 2011; Judge et al. 2011, Canals and Bozinovic 2011). In contrast, individuals that are part of aggregations may be able to delay the closure of their opercula in response to the humid microclimate created by aggregation, thereby maintaining gas exchange with the environment for longer periods of time. If considered at the scale of an individual snail, the relative humidity and temperature of the air surrounding an individual sets the limits to which the water loss by evaporation occurs. For example, among animals that are exposed to high aerial temperatures, those which are subject to lower relative humidity will experience greater water loss at lower thermal differences between body and air than individuals exposed to greater relative humidity (Porter and Gates 1969; Helmuth 1998). This prediction is consistent with the lack of movement reported in snails during dry periods (Emson et al. 2002, Judge et al. 2009). Thus, for a snail forming part of an aggregation, the establishment of a humid microclimate may contribute not only to water conservation but may also reduce its exposure to the dry environment. Here, we evaluate the relationship between the behavioral responses to desiccation stress in a littorinid gastropod, Echinolittorina peruviana, common to the high intertidal south east Pacific shores from Panama to central Chile. At mid latitudes, the temperature of the air and rock substrata often reaches maximal temperatures of over 30 and 38 °C, respectively, during warm summer months (Rojas et al. 2000; Finke et al. 2009; Mellado 2003). Furthermore, during summer, it is common to register two low tide events in the daylight hours, which may prolong the exposure of snails to aerial conditions (Rojas et al. 2000; Finke et al. 2007). Through a series of laboratory trials, tidal emersion conditions with varied relative humidity were simulated, we examined the relationship between two behaviors in E. peruviana, opercular closure and aggregation, while evaluating any benefit of these behaviors during conditions of thermal and desiccation stress. While this approach might not represent a faithful reproduction of the natural environment, it allowed us to recreate, at least in.
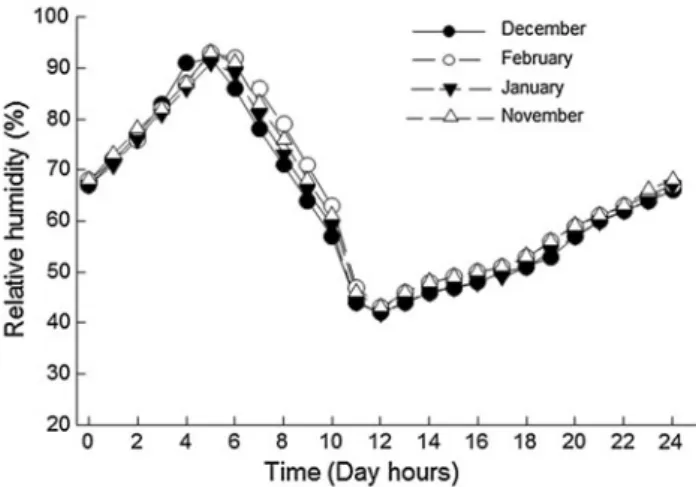
(3) Mar Biol (2013) 160:1119–1126. part, the microclimatic environment experienced by a littorinid during tidal emersion periods. We hypothesized that solitary snails should keep their opercula open for less time than aggregated snails when exposed to thermal stress, but that this relationship should decrease with increasing relative humidity. Rates of water loss and increase in body temperature should be less among aggregated snails in comparison with solitary individuals, although both should vary as a function of environmental humidity.. Materials and methods For each trial, approximately 100 individuals of E. peruviana with similar body size (11.0 ± 3.0 mm) were collected during low tide periods from the high intertidal zone at the wave exposed shore of Las Cruces, Chile (33°350 S; 71°38 W). Isolated and aggregated individuals were chosen randomly from horizontal platforms with similar topographic features. A total 4 collections were carried out between April and June of 2011. Once collected, individuals were transported to the laboratory and acclimated for 3 days in tanks (50 L) in a temperature controlled room (21 °C) and exposed to a 12:12 photoperiod prior to the experiments. In an effort to better simulate the natural environment, in each tank we placed stone plates forming an irregular horizontal surface surrounded by a pool of aerated seawater. The seawater was changed daily, and the emergent stone surface was moistened once a day with a hand sprayer. In the experimental trials, snails were exposed to simulated tidal emersion conditions for a period of 4 h in one of two growth cabinets (Intelligent Artificial Climate box Model MPA-100; Winpark Electronics Co.). The cabinets were maintained with a constant aerial temperature (Ta) of 30 °C; we selected this value considering. Fig. 1 Microclimatic simulation of the daily variation in the relative humidity of the air for the Las Cruces on rock surface (3 cm high), during the summer months. 1121. the mean maximum daily temperatures registered at the collection site (Rojas et al. 2000; Finke et al. 2009). Relative humidity (RH) was held constant at 30, 50, 65, 80 or 90 %. Extreme humidity values used corresponded to the results of simulations of microclimatic conditions that may be experienced by a snail on the upper shores of Las Cruces during a typical midday low tide during the summer months (Fig. 1). The simulations were performed using Niche Mapper software (Natori and Porter 2007), using data collected during the last 10 years by the meteorological station (Campbell CR10X) located in the marine research station of ECIM (Estación Costera de Investigaciones Marinas), Las Cruces, Chile. Within each cabinet, we placed 8 Petri plates (60 mm diameter) into which snails were transferred from the acclimation tanks. Individuals selected for each trial were active (opened operculum) and out of water. Prior to transfer, each Petri plate was moistened with seawater, with the objective that each snail began the study under similar environmental conditions. A Petri plate with one snail was defined as the solitary treatment (60 total individuals) and a plate with six individuals as the aggregated treatment (300 total individuals). Individual snails were used in only once in a single trial. Once snails were placed, experimental units were exposed to five levels of RH (30, 50, 65, 80 or 90 %) following an orthogonal two-way ANOVA design, resulting in 12 replicates by treatment combination for solitary individuals and 10 replicates for aggregated treatments. Within aggregated treatments, only one individual per plate was used as the study subject at time of evaluation. These focal snails were selected only if the individual was surrounded by at least 3 conspecifics by the end of the experimental trial (Fig. 2). For each replicate, we measured the time that focal snails (both solitary and aggregated treatments) maintained their opercula open. The close of the operculum was monitored constantly over the course of the trials using a webcam placed inside the cabinet underneath each group of experimental units. Body temperature (Tb) was recorded during the first hour of the experiment for the two extreme humidity conditions (30 and 90 %), every 9 min, using an infrared thermographic camera (Flir model i40; Fig. 2). Preliminary data collected prior to experimental trials showed that snails reached a stable body temperature after 1 h of exposure to 30 °C, without consequences for survival. This last observation offers further support to the appropriateness of the air temperature selected and the feasibility of estimating rates of temperature change of during the first hour of our experimental trials. The rate of water loss experienced by individuals was then estimated from the difference between initial and final body mass standardized by the initial weight (obtained with a Chyo, JK-180 analytic balance; with precision ± 0.0001 g) and time that the operculum remained open.. 123.
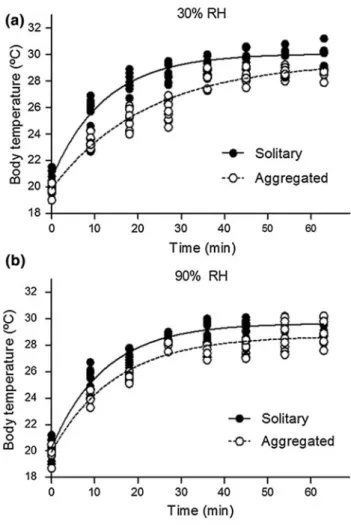
(4) 1122. Mar Biol (2013) 160:1119–1126. Prior to the fitting procedure, the normality assumption was tested using D’Agostino and Pearson omnibus K2 test, and the independence of error was evaluated with a visual inspection of residuals for each data series. As mentioned previously, results were compared by performing a twoway ANOVA, considering aggregation condition (solitary or aggregated individuals) and RH level as fixed factors. We used a type II model in order to reduce the potential effects of having an unbalanced design (10 solitary vs. 12 aggregated) on the estimation of sums of squares for ANOVA (Langsrud 2003). Prior to all analyses, we tested differences in the body mass at the start of the study among experimental groups (aggregated or solitary individuals) using a one-way ANOVA. A slope homogeneity test was performed to evaluate the possible influence of the number of neighbors on the response variables, using the number of conspecifics surrounding the focal snail over the duration of the trial as a covariate. Paired comparisons were performed using the Fisher’s least significant difference test. Assumptions of each test were evaluated before analysis. The time that opercula remained open was transformed using loge(x), and the water loss rate was transformed using the arcsin (Hp). Unless otherwise stated, all statistical analyses were performed using the STATISTICA software (Statsoft v5.0).. A B. Fig. 2 Thermal image of a typical experimental group after 40 min of exposure to an ambient temperature of 30 °C and 30 % relative humidity. The spatial arrangement solitary (a) and aggregated (b) snails is a consequence of the natural displacement of individuals during the treatment. The kinetics of the heat gain in solitary and aggregated individuals exposed to 30 or 90 % RH was characterized by fitting a single exponential model of association with three parameters (y = Y0 ? a(1-exp(-K*x)) (Table 1 for details) using least square mean method (GraphPad Prism 5.0). To test whether solitary and aggregated individuals followed the same heat gain kinetic, we evaluated whether the same parameters fit the best curve for the data points of solitary snails and aggregate within the same RH level using the extra sum of squares F test (GraphPad Prism 5.0).. Results Solitary and aggregated snails exposed to extreme RH values (30 or 90 %) showed differences in kinematic warming (Fig. 3; Table 1). Maximum Tb values were slightly higher among solitary than aggregated individuals; however, differences of the same order were shown in the initial Tb between the two treatment groups, thereby limiting any conclusions as to the effect of aggregation on total. Table 1 Fitted parameters to curve warming of snails exposed to 30 and 90 % relative humidity Parameters. 30 % Solitary. 90 % Aggregate. Solitary. Aggregate. Y0. 20.7 ± 0.2. 19.9 ± 0.2. 20.2 ± 0.2. 19.9 ± 0.2. a. 30.1 ± 0.2. 29.5 ± 0.2. 29.7 ± 0.1. 28.7 ± 0.2. K. 0.08 ± 0.07. 0.05 ± 0.04. 0.08 ± 0.01. 0.06 ± 0.01. Span. 9.4 ± 0.2. 9.6 ± 0.3. 9.4 ± 0.2. 8.8 ± 0.3. Half-time. 8.5. 15.3. 8.5. 10.2. R. 0.96. 0.95. 0.96. 0.94. df. 93. 77. 93. 77. Series comparisons. F (3,170) = 125. p \ 0.0001. F (3,72) = 43.7. p \ 0.0001. 2. F and p values for the statistic F and significance from extra sum of square test used to compare the data series Y0 initial average body temperature, a mean maximal temperature reached, K constant, Span increment in temperature, Half-time time to reach half temperature maximum observed. 123.
(5) Mar Biol (2013) 160:1119–1126. 1123 Table 2 Results of the two-way analysis of variance for the effect of the aggregation (Ag) and the percentage of relative humidity (RH) of environment on the opercular exposure time (time spent open) and weight loss rate in the snail E. peruviana Response variable. Effects. Weight loss rate (mg g-1 min-1). RH. 4. 0.00. 2.00. 0.091. Ag. 1. 0.00. 33.00. 0.001. RH 9 Ag. 4. 0.00. 5.00. 0.001. 100. 0.00. Exposure time (min). df. MS. F. p. RH. 4. 0.13. 1.27. 0.293. Ag. 1. 3.24. 32.12. 0.001. 4. 0.74. 5. 46. 0.001. 100. 0.10. RH 9 Ag Error. Fig. 3 Body temperature warming curves for solitary and aggregated snails exposed to a 30 % and b 90 % relative humidity. Ambient temperature was held constant at 30 °C. See text for details. Tb gain (Table 1). Nevertheless, at 30 % RH, solitary individuals exhibited greater rates of Tb increase than aggregated snails (Table 1; Fig. 3). A similar result was also observed, albeit to a lesser extent, in snails exposed to 90 % RH (Table 1; Fig. 3). Solitary and aggregated snails exhibited differences in the rate of water loss when exposed to simulated tidal emersion conditions with the magnitude of this difference dependent on the prevailing RH (Table 2). Solitary individuals lost water at a greater rate when exposed to lower RH (30 and 50 %) than aggregated snails (Fig. 4). No differences in water loss were observed between solitary or aggregated snails exposed to greater RH (65, 80 and 90 %). Among solitary snails, those exposed to 80 and 90 % RH lost water at a similar rate, but less than snails exposed to 30 and 50 % RH. Solitary snails exposed to 65 % RH experienced less weight loss than snails exposed at 80 % RH (Fig. 4), but similar to loss rates of snails exposed to 30, 50 and 90 % RH. Snails exposed to 30 and 50 % RH no showed significant difference between them (LSD Fisher test, p [ 0.05, Fig. 4). Among aggregated snails,. Fig. 4 a Mean (±SE) loss of body mass due to evaporative water loss and b average time that kept open the operculum of both solitary and aggregated individuals exposed to different levels of relative humidity. Changes in mass are standardized by the initial weight and opened time. Asterisks indicate significant differences (p \ 0.05) between aggregated and solitary individuals exposed to similar relative humidity conditions. (See text to other paired comparisons). differences in water loss were observed only between snails exposed to RH 80 and 90 % (LSD Fisher test, p \ 0.05; Fig. 4), where snails exposed to 80 % RH experienced a slightly greater water loss. Solitary and aggregated individuals also exhibited differences in the time that their opercula remained open. 123.
(6) 1124. under varying RH conditions (Table 2). The opercula of solitary snails remained open for less time than aggregated snails when they were exposed to 30, 50 or 65 % RH (Fig. 4). For 80 and 90 % RH, solitary and aggregated snails did not exhibit differences in the time that the opercula remained open. Among solitary snails, individuals at 30 % RH kept their opercula open for less time compared with snails at 65, 80 or 90 % RH (LSD Fisher test, p \ 0.05; Fig. 4). Solitary snails held at 50 % RH maintained their opercula open for less time than snails held at 80 % RH (LSD Fisher test, p \ 0.05, Fig. 4). No differences were observed in time that solitary individuals maintained their opercula open when exposed to 65, 80 and 90 % RH (LSD Fisher test, p [ 0.05; Fig. 4). Among aggregated snails, open time of the opercula was significantly higher in snails held at 30, 50 and 65 % RH in comparison with snails held at 80 and 90 % RH (LSD Fisher test, p \ 0.05, Fig. 4). No differences were observed among aggregated snails held at 30, 50 and 65 % RH (Fig. 4) or among snails exposed to 80 and 90 % RH (Fig. 4). The number of neighbors that surrounded the focal snail in the aggregated treatment had no effect on the time that the opercula were kept open nor on the water loss rate (exposure time: F (4;40) = 0.37, p = 0.82; water loss: F (4;40) = 0.21, p = 0.93). Finally, initial body mass was similar among all snails (Interaction RH x aggregation treatment: F (4;100) = 0.25, p = 0.91), reducing bias associated with differences in the rate of water loss on initial snail condition.. Discussion Previous research has suggested that the formation of aggregations among littorinid snails may be the result of individual behaviors in response to risk of desiccation, starvation, or predation (Chapman 1995; Stafford et al. 2007; Coleman 2010). During tidal emersion periods, dry substrata restrict the movement of snails and prompt the closing of their operculum (Emson et al. 2002; Judge et al. 2009). Hence, it is possible to assume that for snails exposed to drier, aerial conditions, the amount of time the operculum remains open should be less than when conditions are more wet or humid. In this study, aggregated snails maintained their opercula open for more time than solitary individuals when exposed to drier conditions. Moreover, aggregated individuals held at low RH maintained lower body temperatures and sustained less water loss than solitary individuals. Our findings suggest that while huddling up together, snails apparently are less susceptible to harsh environmental conditions, being able to maintain gaseous exchange with the environment for more time than solitary snails under periods of desiccation stress.. 123. Mar Biol (2013) 160:1119–1126. In addition to the challenges of desiccation stress, the body temperature of snails in the upper intertidal fringe is subjected to daily oscillations in air temperature, imposing unfavorable conditions and negative consequences for physiological and locomotory performance of ectotherm organisms. For littorinids, there is a described suppression of metabolic rate in response to heating, which allows entry to short-term metabolic diapause, thus conserving energy at higher temperatures to offset lifelong constraints on energy gain due to daily thermal oscillations (McMahon et al. 1995; Marshall and McQuad 2011). As part of this metabolic adjustment, the performance curve follows a bimodal shape with temperature (Marshall and McQuad 2011). Field observations and laboratory experimentation indicate that snails are not static within aggregations, but move around among individuals with potential displacement only halted when the snails have closed their opercula (Chapman 1995; Rojas et al. 2000). In our study, an open operculum is not synonymous with locomotory activity; hence, we cannot estimate the energetic gain associated with forming part of an aggregation. Instead, our results suggest that aggregation behavior in snails may aid individuals to cope with the climatic variability typical of tidal emersion periods. Furthermore, our findings should promote further debate and research on the potential importance of aggregation for metabolic performance. For instance, a reduction in the heat transfer rate between a snail and its environment related to a more humid microclimate, together with the potential benefit for the heat-shock protein system of a prolonged oxygen supply, could be two interesting points of interaction in the physiology–behavior relationship for thermal control. However, whether the increase in time that opercula are kept open has a direct, positive consequence for physiological performance during low tide requires further study. Indeed, higher levels of heat-shock proteins (Hsp70) were described in hydrated snails in comparison with snails on dry substrata in the field (Judge et al. 2011). Historically, evidence espousing the role of aggregation behavior in water conservation has been largely anecdotal, but what is clear is the size of aggregations in intertidal communities and the frequency with which they occur vary greatly in space and time (Chapman 1995; Chapman and Underwood 1996; Rojas et al. 2000). Although aggregations are clearly beneficial under thermally stressful conditions characterized by low RH, snails held at higher RH experienced increasingly similar rates of water loss and body temperature increases, regardless of whether or not they were surrounded by conspecifics. Along the northcentral coast of Chile, where E. peruviana are frequently observed as both solitary individuals and as part of dense aggregations, the tendency to aggregate may be a function of RH during low tide or specific microclimate. This does not necessarily imply that the aggregations of gastropods.
(7) Mar Biol (2013) 160:1119–1126. conform to a group behavior. Indeed, these aggregations are commonly observed near rock cracks and crevices; thus, the observation of aggregations may be simply the natural consequence of a common preference of snails to avoid dry substrata and to congregate where substrata topography creates a humid microclimate (Chapman 1994; Stafford and Davies 2005; Jackson 2010; Chapperon and Seuront 2011a, b; Stafford et al. 2012). Aggregations may also increase competition for food among conspecifics, thus snails may only be compelled to congregate when either food supply is abundant (Mak and Williams 1999) or thermal or desiccation stress is particularly harsh. Future field studies of aggregation behavior might also incorporate the potential effects of specific behavioral cues or individual risk factors involved in promoting and maintaining aggregations. Finally, the effects of topographic features and their influence on microhabitat conditions are likely to influence the distribution and behavior of snails (Underwood and Chapman 1992; Jackson 2010). In conclusion, our results suggest a complementary role of two behaviors that confer physiological benefits when confronted with extreme physical conditions experienced during emersion periods. We concur with Kearney et al. (2009), who emphasize the importance of behavior in buffering the impacts of global climate change, arguing that behavior is an important and missing element from models of climatic change and predictions of impacts on biodiversity. Similarly, Somero (2011) concludes that through a mechanistic understanding of both sublethal and lethal stresses and the differences that exist within and among species in their capacities to respond to these stresses (e.g., aggregation behavior), a foundation can be constructed for developing predictions about the probability of success or failure of organisms, populations and species to cope with climatic change. Overall, the results of our study suggest the need for an integrative focus of factors that contribute to thermal regulation in order to improve our understanding of thermal physiology of intertidal organisms, a perspective that would allow us to accomplish more realistic forecasts addressing the potential consequences of global warming on the distributions of species. Acknowledgments 1120276.. Funded by LINC-Global and FONDECYT. References Bates AE, Tunnicliffe V, Lee RW (2005) Role of thermal conditions in habitat selection by hydrothermal vent gastropods. Mar Ecol Prog Ser 305:1–15 Bozinovic F, Calosi P, Spicer JI (2011) Physiological correlates of geographic range in animals. Ann Rev Ecol Evol Syst 42:155– 179. 1125 Canals M, Bozinovic F (2011) Huddling behavior as critical phase transition triggered by low temperatures. Complexity 17:35–43 Chapman MG (1994) Small-scale patterns of distribution and sizestructure of the intertidal littorinid Littorina unifasciata (Gastropoda: Littorinidae) in New South Wales. Aust J Mar Fresh Res 45:635–652 Chapman MG (1995) Aggregation of the littorinid snail Litorina unifasciata in New SouthWales, Australia. Mar Ecol Prog Ser 126:191–202 Chapman MG, Underwood AJ (1996) Influences of tidal conditions, temperature and desiccation on patterns of aggregation of the high-shore periwinkle, Littorina unifasciata, in New South Wales, Australia. J Exp Mar Biol Ecol 196:213–223 Chapperon C, Seuront L (2011a) Behavioral thermoregulation in a tropical gastropod: links to climate change scenarios. Glob Change Biol 17:1740–1749 Chapperon C, Seuront L (2011b) Space-time variability in environmental thermal properties and snail thermoregulatory behavior. Funct Ecol 25:1040–1050 Coleman RA (2010) Limpet aggregation does not alter desiccation in the limpet Cellana tramoserica. J Exp Mar Biol Ecol 386: 113–118 Coleman RA, Goss-Custard JD, Sarah EA, Durell LV, Hawkins SJ (1999) Limpet Patella spp. Consumption by oystercatchers Haematopus ostralegus: a preference for solitary prey items. Mar Ecol Prog Ser 183:253–261 Dahlhoff EP, Buckley BA, Menge BA (2001) Physiology of the rocky intertidal predator Nucella ostrina along an environmental stress gradient. Ecology 82:2816–2829 Denny MW, Miller LP, Harley CDG (2006) Thermal stress on intertidal limpets: Long-term hindcasts and lethal limits. J Exp Biol 209:2420–2431 Dong YW, Yu SS, Wang QL, Dong SL (2011) Physiological responses in a variable environment: relationships between metabolism, Hsp and thermotolerance in an intertidal-subtidal species. PLoS ONE 6:e26446 Emson RH, Morritt D, Andrews EB, Young CM (2002) Life on a hot dry beach: behavioural, physiological, and ultrastructural adaptations of the littorinid gastropod Cenchritis (Tectarius) muricatus. Mar Biol 140:723–732 Finke G, Navarrete S, Bozinovic F (2007) Tidal regimes of temperate coasts and their influences on aerial exposure for intertidal organisms. Mar Ecol Prog Ser 343:57–62 Finke G, Bozinovic F, Navarrete S (2009) A mechanistic model to study the thermal ecology of a southeastern pacific dominant intertidal mussel and implications for climate change. Physiol Biochem Zool 82:303–313 Firth L, Knights A, Bell S (2011) Air temperature and winter mortality: implications for the persistence of the invasive mussel, Perna viridis in the intertidal zone of the south-eastern United States. J Exp Mar Biol Ecol 400:250–256 Garrity SD (1984) Some adaptations of gastropods to physical stress on a tropical rocky shore. Ecology 65:559–574 Gendron RP (1977) Habitat selection and migratory behaviour of the intertidal gastropod Littorina littorea (L.). J Anim Ecol 46:79–92 Helmuth B (1998) Intertidal mussel microclimates: predicting the body temperature of a sessile invertebrate. Ecol Monogr 68:51– 74 Helmuth B, Kingsolver JG, Carrington E (2005) Biophysics, physiological ecology, and climate change: does mechanism matter? Ann Rev Physiol 67:177–201 Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Ann Rev Ecol Evol Syst 37:373–404. 123.
(8) 1126 Helmuth B, Yamane L, Lalwani S, Matzelle A, Tockstein A, Gao N (2011) Hidden signals of climate change in intertidal ecosystems: what (not) to expect when you are expecting. J Exp Mar Biol Ecol 400:191–199 Hofman GE (1999) Ecologically relevant variation in induction and function of heat shock proteins in marine organism. Am Zool 39:899–900 Hughes R (1986) A functional biology of marine gastropods. Croom Helm, London Jackson AC (2010) Effects of topography on the environment. J Mar Biol Assoc UK 90:169–192 Judge ML, Duell R, Burriesci L, Moarsi W (2009) Life in the supralittoral fringe: microhabitat choice, mobility and growth in the tropical perwinkle Cenchritis (= Tectarius) muricatus (Linneaus, 1758). J Exp Mar Biol Ecol 369:148–154 Judge ML, Botton ML, Hamilton MG (2011) Physiological consequences of the supralittoral fringe: microhabitat temperature profiles and stress protein levels in the tropical periwinkle Cenchritis muricatus (Linneaus, 1758). Hydrobiol 675:143–156 Kearney MR, Shine R, Porter WP (2009) The potential for behavioral thermoregulation to buffer ‘‘cold-blooded’’ animals against climate change. Proc Natl Acad Sci USA 106:3835–3840 Kordas RL, Harley C, O’Connor MI (2011) Community ecology in a warming world: the influence of temperature on the interspecific interactions in marine system. J Exp Mar Biol Ecol 400:218–226 Langsrud Ǿ (2003) ANOVA for unbalanced data: use type II instead of type III sum of square. Stat Comput 13(163):167 Lee SL, Lim SSL (2009) Vertical zonation and heat tolerance of three littorinid gastropods on a rocky shore at Tanjung Chek Jawa, Singapore. Raff Bull Zool 57:10 Mak YM, Williams GA (1999) Littorinids control high intertidal biofilm abundance on tropical, Hong Kong rocky shores. J Exp Mar Biol Ecol 233:81–94 Marshall DJ, McQuad CD (2011) Warming reduce metabolic rate in marine snail: adaptation to fluctuating high temperatures challenges the metabolic theory of ecology. Proc R Soc B 278: 281–288 McMahon RF, Russellhunter WD, Aldridge DW (1995) Lack of metabolic temperature compensation in the intertidal gastropods, Littorina saxatilis (Olivi) and Littorina obtusata (L). Hydrobiologia 309:89–100 Mellado O (2003) Sectorización Climático-Habitacional de las Regiones de Valparaı́so y Metropolitana. Bol INVI 18:35–59 Miller LP, Denny MW (2011) Importance of behavior and morphological traits for controlling body temperature in littorinid snails. Biol Bull 220:209–223 Muñoz JLP, Finke GR, Camus PA, Bozinovic F (2005) Thermoregulatory behavior, heat gain and thermal tolerance in the. 123. Mar Biol (2013) 160:1119–1126 periwinkle Echinolittorina peruviana in central Chile. Comput Biochem Physiol A 142:92–98 Natori Y, Porter WP (2007) Model of Japanese serow (Capricornis crispus) energetics predicts distribution on Honshu, Japan. Ecol Appl 17:1441–1459 Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monogr 39:227–244 Rojas JM, Fariña J, Soto R, Bozinovic F (2000) Variabilidad geográfica en la tolerancia térmica y economı́a hı́drica del gastrópodo intermareal Nodilittorina peruviana (Gastropoda: Littorinidae, Lamarck, 1822). Rev Chil Hist Nat 73:543–552 Scavia D, Field JC, Boesch DF, Buddemeier RW, Burkett V, Cayan DR, Fogarty M, Harwell MA, Howarth RW, Mason C, Reed DJ, Royer TC (2002) Climate change impacts on U.S. coastal and marine ecosystems. Estuaries 25:15 Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comput Biol 42:780–789 Somero GN (2011) Comparative physiology: a ‘‘crystal ball’’ for predicting consequences of global change. Am J Physiol Regul Integr Comp Physiol 302:R1–R14 Stafford R, Davies MS (2005) Examining refuge location mechanisms in intertidal snails using artificial life simulation techniques. LNAI 3630:520–529 Stafford R, Davies MS, Williams GA (2007) Computer simulations of high shore littorinids predict small-scale spatial and temporal distribution patterns on rocky shores. Mar Ecol Prog Ser 342:151–161 Stafford R, Davies MS, Williams GA (2012) Misinterpreting the potential benefits of aggregation for reducing desiccation in the intertidal: a simple analogy. Mar Ecol 33:512–515 Tomanek L (2002) The heat-shock response: its variation, regulation and ecological importance in intertidal gastropods (genus Tegula). Integr Comput Biol 42:797–807 Tomanek L (2010) Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J Exp Biol 213:971–979 Underwood AJ, Chapman MG (1992) Experiments on topographic influences on density and dispersion of Littorina unifasciata in New South Wales. In: Grahame J, Mill PJ, Reid DG (eds) Proceedings of the third international symposium on Littorinid biology. The Malacological Society of London, London, pp 181–195 Urian AG, Hatle JD, Gilg MR (2011) Thermal constraints for range expansion of the invasive green mussel, Perna viridis, in the southeastern United States. J Exp Zool 315A:12–21.
(9) Copyright of Marine Biology is the property of Springer Science & Business Media B.V. and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use..
(10)
Figure

Documento similar
By levels of educational attainment, differences in returns also account for most of the gender wage gap in the sub-sample of individuals who achieve a level up to secondary
With the interest to investigate the potential role of gremlin in the kidney in physiological and pathological conditions in vivo, we generated viable transgenic (TG) mice
The increase in dry weight (Figure 1A) and dry matter (Figure 2A) in lettuce plants exposed to long photoperiods is consistent with the hypothesis that longer
in 1 /3 of the cases, a contribution of less than 5% to the resonance of the ground state at an excitation time of 4 s was estimated. Therefore, a contribution of the isomeric state
When consumers do not do everything in their power to take care of the environment and see the benefits that could be achieved with everyday actions (using rechargeable
The expansionary monetary policy measures have had a negative impact on net interest margins both via the reduction in interest rates and –less powerfully- the flattening of the
Jointly estimate this entry game with several outcome equations (fees/rates, credit limits) for bank accounts, credit cards and lines of credit. Use simulation methods to
In our sample, 2890 deals were issued by less reputable underwriters (i.e. a weighted syndication underwriting reputation share below the share of the 7 th largest underwriter