Chikungunya virus infection: an overview
Claudia Caglioti, Eleonora Lalle, Concetta Castilletti, Fabrizio Carletti, Maria Rosaria Capobianchi, Licia Bordi
Laboratory of Virology, “L. Spallanzani” National Institute for Infectious Diseases, Rome, Italy
INTRODUCTION
Chikungunya virus (CHIKV), an arbovirus trans-mitted by mosquito vectors, is an alphavirus be-longing to the Togaviridaefamily. Alphaviruses are small spherical enveloped viruses, with a 60-70 nm diameter. The genome is a single-strand RNA molecule of positive polarity, encoding four non structural (nsP1-4) and three structural pro-teins (C, E1, E2). Viral replication is initiated by attachment of the viral envelope to host cell
re-Corresponding author Maria Rosaria Capobianchi Laboratory of Virology Padiglione Baglivi
National Institute for Infectious Diseases INMI “L. Spallanzani”
Via Portuense, 292 - 00149 Rome, Italy E-mail: maria.capobianchi@inmi.it
ceptors (Strauss and Strauss, 1994), followed by clathrin-mediated endocytosis of the attached par-ticle (Lee et al., 2013), low pH-mediated membrane fusion and delivery of the viral nucleocapsid into the cytoplasm (Sorisseau et al.,2007). To date no CHIKV interacting protein has been characterized, but in a very recent study, Wintachai et al. identi-fied prohibitin as CHIKV-binding protein ex-pressed by microglial cells (Wintachai et al., 2012). The replication cycle is fast, taking around 4 hours. Alphaviruses are sensitive to dissecation and to temperatures above 58°C (Strauss and Strauss, 1994; Khan et al., 2002). About 30 species of arthro-pod-borne viruses are included in the alphavirus genus, antigenically classified into 7 complexes. These viruses are widely distributed throughout the world, with the exception of Antarctica. Besides CHIKV, several arthropod-transmitted alphaviruses cause human disease, characterized by similar clinical presentation: Barmah Forest Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus belonging to the Togaviridaefamily, first isolated in Tanzania in 1952. The main vectors are mosquitoes from the Aedesspecies. Recently, the establishment of an en-velope mutation increased infectivity for A. albopictus. CHIKV has recently re-emerged causing millions of infections in countries around the Indian Ocean characterized by climate conditions favourable to high vector density. Importation of human cases to European regions with high density of suitable arthropod vectors (such as A. albopictus) may trig-ger autochthonous outbreaks. The clinical signs of CHIKV infection include non-specific flu-like symptoms, and a char-acteristic rash accompanied by joint pain that may last for a long time after the resolution of the infection. The death rate is not particularly high, but excess mortality has been observed in concomitance with large CHIKV outbreaks. De-regulation of innate defense mechanisms, such as cytokine inflammatory response, may participate in the main clin-ical signs of CHIKV infection, and the establishment of persistent (chronic) disease. There is no specific therapy, and prevention is the main countermeasure. Prevention is based on insect control and in avoiding mosquito bites in en-demic countries. Diagnosis is based on the detection of virus by molecular methods or by virus culture on the first days of infection, and by detection of an immune response in later stages. CHIKV infection must be suspected in patients with compatible clinical symptoms returning from epidemic/endemic areas. Differential diagnosis should take into ac-count the cross-reactivity with other viruses from the same antigenic complex (i.e. O’nyong-nyong virus).
KEY WORDS:CHIKV, Arbovirus, Virus dissemination, Immunopathogenesis, Geographic distribution, Diagnosis, Treatment and prevention.
SUMMARY
(BFV) and Ross River viruses (RRV) (Oceania), O’nyong-nyong (ONNV) and Semliki Forest virus-es (SFV) (Africa), Mayaro (South America), Sindbis (SINV) and Sindbis-like viruses (Africa, Asia, Scandinavia and Russia) (Taubiz et al., 2007). Chikungunya fever (CHIKF) derives its name from Makonde, a language spoken in south Tanzania, and means “that which bends up”, re-ferring to the posture of patients afflicted with severe joint pain characterizing this infection. First isolated in Tanzania in 1952 (Robinson, 1955), CHIKV attracted worldwide attention when it caused a massive outbreak in the Indian Ocean islands (Enserik, 2006). Since 1952, CHIKV has caused a number of epidemics, both in Africa and in Southeast Asia, many of them in-volving hundreds of thousands of people. After a few years of relative dormancy in La Réunion Island, CHIKV transmission has restarted, re-newing concerns about the possibility of renewed autochthonous transmission in Mediterranean countries.
GEOGRAPHIC DISTRIBUTION
CHIKF has an epidemiological pattern with both sporadic and epidemics cases in West Africa, from Senegal to Cameroun, and in many other African countries (Democratic Republic of Congo, Nigeria, Angola, Uganda, Guinea, Malawi, Central African Republic, Burundi, and South Africa). Moreover, many epidemics occurred in Asia (Burma, Thailand, Cambodia, Vietnam, India, Sri Lanka, Timor, Indonesia, and the Philippines) in the 1960s and in the 1990s (Pialoux et al.,2007; Jain et al., 2008).
Major epidemics appear and disappear cyclical-ly, usually with an inter-epidemic period rang-ing from 7 to 20 years. The huge outbreak that increased concern about CHIKV started in Kenya in 2004, where the seroprevalence rates reached 75% in Lamu island (Pialoux et al., 2007), before reaching the Comores, Seychelles, and Mauritius islands. The virus reached La Réunion island in March-April 2005, probably
as a result of importation of cases among immi-grants from the Comores and rapidly spread to several countries in the Indian Ocean and India (Enserik, 2006; Mavalankar et al., 2007). Compared to earlier outbreaks, this episode was massive, occurred in highly medicalized areas such as La Réunion, and had very significant economic and social impact. Since the beginning of the outbreak in the Indian Ocean region, more than 1,000 imported CHIKV cases have been de-tected among European and American travellers returning from the affected areas (Fusco et al., 2006; Taubiz et al., 2007), giving rise, in 2007, to the first autochthonous (human-to-mosquito-to-human transmission) European outbreak in Italy (Rezza et al., 2007; Charrel and de Lambellerie, 2008). During the period December 2006-July 2009, no confirmed cases were detected on La Réunion and Mayotte Islands, but new outbreaks were reported in Madagascar. After a few years of relative dormancy in La Réunion, CHIKV transmission restarted in 2009 and 2010, lead-ing to re-importation to Europe (May 2010) (D’Ortenzio et al., 2011).
During the last three years (2011-2013) concerns about Chikungunya outbreaks arose again due to increasing number of CHIKV infections, start-ing from 2011, when a massive outbreak with more than 11,000 cases occurred in the Republic
of Congo (Brazzaville) (ProMED-mail:
20110613.1806). During 2012, 29 cases of CHIKV infection were reported in India (Rajasthan) (ProMED-mail: 20120716.1203694), and two ad-ditional outbreaks were recorded: one in Cambodia, with almost 1,500 cases (ProMED-mail: 20120920.1303166) and one in the main is-land of Papua New Guinea, with a total of 633 suspected cases (ProMED-mail: 20121010. 1335814); Bali has also had sporadic outbreaks (ProMED-mail: 20130320.1594512). In Samar (Philippines) 600 cases were recorded in 2012, but in 2013 the infection rate has been increas-ing, with 500 cases recorded until March; these numbers appear to be increasing day by day (ProMED-mail: 20130128.1518853). Considering the capacity of CHIKV to emerge, re-emerge, and quickly spread in novel areas, heightened sur-veillance and preparedness seem to be a priori-ty. In particular, travellers act as carriers who in-advertently ferry pathogens between countries. They can thus serve as a sentinel population
pro-viding information on the emergence or emer-gence of an infectious pathogen in a source re-gion, and can be used to map the location, dy-namics and movement of pathogenic strains (Pistone et al., 2009).
The geographic range of CHIKV is mainly in Africa, Asia and Australia (Figure 1).
PHYLOGENESIS
Three lineages of CHIKV, with distinct genotyp-ic and antigengenotyp-ic characteristgenotyp-ics, have been iden-tified. Isolates that caused the 2004-06 Indian Ocean outbreak form a distinct cluster within the large eastern/central Africa (ECSA) phylogenetic group, in addition to the Asian and west African phylogenetic groups (Powers et al., 2000; Schuffenecker et al.,2006).
The divergence of each distinct lineage reflects, to some extent, the path of global transmission and occasional outbreaks. According to phyloge-netic analysis performed by Volk and colleagues (2010), the currently circulating CHIKV strains have an ancestor that existed within the last 500 years. Interestingly, despite their close geographic distance, the two African lineages did not cluster together, indicating limited genetic exchange be-tween the two lineages in Africa. The only ex-ception was a 1963 bat isolate from Senegal, which grouped in the ECSA clade. This finding is the first to suggest that the main West African and ECSA lineages may overlap spatially in the enzootic cycle, at least occasionally (Volk et al.,
2010).
Moreover, phylogenetic analysis of CHIKV strains circulating in A. albopicus-human transmission cycles, obtained during outbreaks, identified the independent acquisition of a common mutation in E1 glycoprotein (E1gp), namely A226V, in strains isolated from different geographic regions (Schuffenecker et al., 2006; de Lambellerie et al.,
2008a). This mutation, together with M269V and D284E E1gp mutations, have been described as molecular signatures of the Indian Ocean out-break (Arankalle et al., 2007; Tsetsarkin et al.,
virus adaptation to the mosquito vector species (see below). Together with the lack of herd im-munity, this might explain the abrupt and esca-lating nature of the La Réunion outbreak. The A226V mutation was clearly demonstrated to in-crease viral fitness in the A. albopictus vector (Vazeille et al., 2007; Tsetsarkin et al.,2007) that, in turn, may expand the potential for CHIKV to diffuse to the Americas and Europe, due to the
widespread distribution of this vector, particu-larly in Italy (Knudsen, 1995). In a previous pa-per we characterized 7 viral isolates (5 imported and 2 autochthonous cases) with respect to the molecular E1 signatures of the Indian Ocean Outbreak, particularly the A226V mutation. These isolates had been obtained from 3 travellers re-turning from Mauritius in 2006, 2 rere-turning from India in 2006 and 2007, and 2 autochthonous
es that occurred during the 2007 Italian outbreak (Bordi et al., 2008) (Figure 2).
All the strains isolated in Italy, both imported and autochthonous, displayed two molecular signa-tures of the Indian Ocean outbreak (M269V and D284E). The A226V mutation was present in all imported and autochthonous cases, with the ex-ception of the isolate imported from the Indian subcontinent in 2006. The absence of this muta-tion in the isolate imported in 2006 from India was in agreement with published data (Arankalle
et al., 2007), and with available GenBank se-quence data indicating that the virus strains cir-culating in India in 2006 lacked this mutation. The presence of A226V in the isolate imported from India in July 2007 and in the isolates from the 2007 Italian outbreak (originating from a case imported from India) supports the view that the virus envelope sequence of strains from India changed over time, acquiring the E1 mutation as-sociated with enhanced fitness in A. albopictus
after 2006. So it appears that the acquisition and fixation of the A226V mutation may be a com-mon pathway of Chikungunya explosion in epi-demic areas, in a parallel interplay with the mos-quito vector dynamics. It is noteworthy that the outbreak in Singapore, where the A226V muta-tion was absent, was rapidly controlled.
VECTOR AND RESERVOIR
Two distinct transmission cycles have been well documented for CHIKV: an enzootic sylvatic cy-cle and an endemic/epidemic urban cycy-cle. The African sylvatic cycle likely involves several ar-boreal Aedes mosquitoes species as vectors (A. furcifer, A. vittatus, A. fulgens, A. luteocephalus, A. dalzieli, A. vigilax, A. camptorhynchites)and non-human primates as reservoir/amplifying hosts. In Africa, the enzootic transmission cycle can spill over to infect people who live nearby, and en-zootic mosquito vectors may be involved in in-ter-human transmission during small outbreaks.
A. furcifer, probably a principal enzootic vector, is known to enter human villages (Diallo, 1999), where it presumably transmits the virus from monkeys to humans (Peyrefitte et al., 2007; Peyrefitte et al., 2008). Endemic/epidemic trans-mission cycles were established when the virus was introduced into Asia around 1950, and into
the Indian Ocean region, India and then Southeast Asia since 2005. As previously stated, a mutation in the E1gp gene, that results in the A226V amino acid substitution, dramatically in-creased the infectivity of some epidemic strains for an alternative urban vector, A. albopictus
(ProMED archive 20100926.3495). Therefore, the urban transmission cycle relies only on A. aegyp-tiand/or A. albopictus, anthropophilic vectors that can initiate human-mosquito-human transmis-sion, and human amplification hosts. This en-demic/epidemic cycle results in high levels of hu-man exposure to mosquito transmission, partic-ularly because these vectors live in close proxim-ity to people. The behaviour and ecology of A. ae-gypti,in particular, are ideal for epidemic trans-mission because adult females prefer to feed on humans, often take several blood meals during a single gonotrophic cycle, oviposit in artificial con-tainers as their preferred larval sites, and rest in-side houses with ready access to human hosts (Weaver et al.,2012).
tem-perature and humidity are achieved easily and naturally.
Human beings serve as the main CHIKV reser-voir during epidemic periods. In Africa some an-imals (monkeys, rodents, and birds) constitute the virus reservoir during non-epidemic periods, sustaining virus circulation in the environment in the absence of human cases. Outbreaks might occur in monkeys when herd immunity is low; the animals develop viremia but no pronounced physical manifestations (Wolfe et al.,2001; Inoue
et al.,2003). An animal reservoir has not been identified in Asia, where humans appear to be the only host.
TREATMENT AND PREVENTION
There are no specific drugs against CHIKV and patients are symptomatically treated with non-steroidal anti-inflammatory drugs, fluids, and medicines to relieve symptoms of fever and aching, such as ibuprofen, naproxen, acetamino-phen, or paracetamol. Steroids have occasional-ly been used but their efficacy was not significant (Taubitz et al.,2007). Some time ago chloroquine, a drug useful for prophylaxis and treatment of malaria, showed promising results for treating chronic Chikungunya arthritis (Brighton, 1984), even if a further trial conducted on La Réunion Island proved that there was no justification for the use of chloroquine to treat acute Chikungunya disease (de Lamballerie et al.,
2008b); overall, the usefulness of chloroquine treatment remains unclear. Ribavirin (200 mg twice a day for seven days) given to patients who continued to have crippling lower limb pains and arthritis for at least two weeks after a febrile episode, seems to be effective against CHIKV, leading to faster resolution of joint and soft tissue manifestations (Ravichandran and Manian, 2008). Briolant and colleagues (2004) screened various active antiviral compounds against virus-es of the alphavirus genus in vitro and demon-strated that 6-azauridinet was more effective than ribavirin against CHIKV. Moreover, the combi-nation of interferon (IFN)-α2b and ribavirin had a synergistic antiviral effect on CHIKV (Briolant
et al., 2004). Since inhibitors of monocyte chemo-taxis can greatly alleviate alphavirus-induced arthritides in mice (Rulli et al., 2009) the use of
in-hibitors of chemokine pathways associated with monocyte/macrophage recruitment may be a promising approach in humans, to be further ex-plored.
It is widely recognized that passive vaccination is an appropriate preventive and therapeutic option for many viral infections in humans, including those spread by viral vertical transmission, espe-cially when no alternative therapy is available (Dessain et al., 2008). CHIKV infection seems to elicit long-lasting protective immunity, and ex-periments performed using animal models have shown a partial cross-protection among CHIKV and other alphaviruses (Hearn and Rainey, 1963; Edelman et al.,2000). Since human polyvalent im-mune globulins, purified from plasma samples ob-tained from donors in the convalescent phase of CHIKV infection, exhibited high neutralizing ac-tivity in vitroand a powerful prophylactic and ther-apeutic efficacy against CHIKV infection in in vi-vomouse models (Couderc et al., 2009), it could be used in humans for prevention and treatment, es-pecially in individuals at risk of severe CHIKV dis-ease, such as neonates born to viremic mothers and adults with underlying conditions. Polyclonal immune globulins present the advantage of a broad reactivity but the therapeutic intervention is limited, due to the short viremia in the acute phase of CHIKV infection: thus the only benefit this treatment has to offer would be to help reducing viremia faster (Kam et al.,2009). As an alternative approach, more specific human monoclonal an-tibodies (MAbs) could be used. In a recent study two unique human MAbs, specific for the CHIKV E1gp, strongly and specifically neutralized CHIKV infection in vitro(Warter et al.,2011).
To date a number of CHIKV vaccines have been developed, but none have been licensed. While a number of significant questions remain to be ad-dressed related to vaccine validation, such as the most appropriate animal models (species, age, immune status), the dose and route of immu-nization, the potential interference from multi-ple vaccinations against different viruses, and lastly, the practical cost of the vaccine, since most of the epidemic geographical regions belong to the developing countries, there is real hope that a vaccine to prevent this disease will not be too long in arriving.
have been tested in humans and animals with varying success. Several vaccine strategies have been undertaken:
1. whole inactivated virus preparation; 2. attenuated live vaccines;
3. recombinant proteins or virus like particles; 4. DNA vaccination.
Due to the ease of preparation, the first developed vaccines were formulations of whole-virus grown in cell cultures and inactivated either by forma-lin or tween-ether (Harrison et al., 1967; Eckels et al., 1970; Harrison et al.,1971; White et al.,1972). Further vaccines focused on attenuated strains of CHIKV obtained after serial passages in cell cultures (Levitt et al.,1986; Edelman et al.,2000). One of these promising candidates is TSI-GSD-218, a serially passaged and plaque-purified live CHIKV vaccine, tested for safety and immuno-genicity in human Phase II trials by the US Army Medical Research Institute (Edelman et al.,2000). Some chimeric candidate vaccines were
devel-oped using either Venezuelan Equine
Encephalitis virus (VEEV) attenuated vaccine strain TC-83, a naturally attenuated strain of Eastern Equine Encephalitis virus, or SINV as a backbone and the structural protein genes of CHIKV. Vaccinated mice were fully protected against disease and viremia after CHIKV chal-lenge (Wang et al., 2008). Traditional attenuation approaches, relying on cell culture passages, typ-ically result in attenuation that depends only on small numbers of attenuating point mutations. In addition to the risk of reactogenicity, attenua-tion based on small numbers of mutaattenua-tions can also result in residual alphavirus infectivity for mosquito vectors. This risk, underscored by the isolation of the TC-83 VEEV vaccine strain from mosquitoes in Louisiana during an equine vacci-nation campaign designed to control the 1971 epidemic (Pedersen et al.,1972), is especially high when a vaccine that relies on a small number of point mutations is used in a non-endemic loca-tion that could support a local transmission cycle. In 2012, the United States Army developed and tested a live attenuated strain of CHIKV,
CHIKV181/25 for vaccine application.
CHIKV181/25 demonstrated an excellent im-munogenic profile, however, transient arthralgia was observed in about 8% of vaccine recipients. Sharma and colleagues tried to inactivate CHIKV181/25 with 1,5 iodonapthyl azide (INA),
a photoactive hydrophobic azide molecule that they used in a previous study (Sharma et al.,
2007) to completely inactivate VEEV, in addition to UV irradiation. The INA-inactivated CHIKV181/25 formulation may address the issue of residual virulence associated with live attenu-ated CHIKV181/25, but the INA-inactivation re-sults in a relatively weaker binding capacity of CHIKV181/25 to the neutralizing polyclonal an-ti-CHIKV E2 glycoprotein (E2gp) so that further investigations are necessary (Sharmaet al.,2012). Alternative genetic strategies such as viral chimeras offer the promise of more stable atten-uation (Kennedy et al.,2011). For instance, a re-cent study showed that chimeric alphaviruses, encoding CHIKV-specific structural genes (but no structural or nonstructural proteins capable of interfering with development of cellular an-tiviral response), induce protective immune re-sponse against subsequent CHIKV challenge (Wang et al., 2011).
A novel CHIKV vaccine candidate, CHIKV/IRES (internal ribosome entry site), was generated by manipulation of the structural protein expression of a wt-CHIKV strain via the encephalomyocardi-tis virus IRES, and exhibited a high degree of murine attenuation that was not dependent on an intact IFN type I response, highly attenuated and efficacious after a single dose (Plante et al.,2011). Another approach, recently undertaken by Akata and colleagues (2010), was the use of virus-like particles (VLPs) expressing CHIKV structural pro-teins that resemble replication-competent al-phaviruses (Akahata et al.,2010). Immunization of monkeys with these VLPs elicited neutralizing antibodies against envelope proteins from different CHIKV strains that could confer passive protec-tion against lethal CHIKV challenge into new mice. The last frontier in the approach of CHIKV vac-cine design is the DNA vacvac-cine strategy. An adap-tive constant-current electroporation technique
was used to immunize mice (Muthumani et al.,
2008) and rhesus macaques (Mallilankaraman et
with promising results (Kumar et al.,2012). Since a vaccine is not currently available, protection against mosquito bites and vector control are the main preventive measures. Individual protection relies on the use of mosquito repellents and meas-ures to limit skin exposure to mosquitoes. Bednets should be used during the night in hos-pitals and day-care facilities but Aedes mosqui-toes are active all-day long. Control of both adult and larval mosquito populations uses the same model as for dengue and has been relatively ef-fective in many countries and settings. Breeding sites must be removed, destroyed, frequently emptied, and cleaned or treated with insecticides. Control of A. aegyptihas rarely been achieved and never sustained (Reiter et al.,2006). Recent data show the different degrees of insecticide resist-ance in A. albopictusand A. aegypti(Cuiet al.,
2006). Large-scale prevention campaigns using dichlorodiphenyltrichloroethane have been ef-fective against A. aegyptibut not A. albopictus. However, vector control is an endless, costly, and labor-intensive measure and is not always well accepted by local populations, whose coopera-tion is crucial. Control of CHIKV infeccoopera-tion, oth-er than use of drugs for treatment of disease, de-velopment of vaccines, individual protection from mosquitoes and vector control programs, also in-volves surveillance that is fundamental for early identification of cases and quarantine measure-ment. A model used in investigation of the trans-mission potential of CHIKV in Italy has proven useful to provide insight into the possible impact of future outbreaks in temperate climate regions and the effectiveness of the interventions per-formed during the outbreak (Poletti et al., 2011).
CLINICAL MANIFESTATIONS
After infection with CHIKV, there is a silent in-cubation period lasting about 2-4 days (range 1-12 days) (Lam et al.,2001). Clinical onset is abrupt, with high fever, headache, back pain, myalgia, and arthralgia; the latter can be intense, affecting mainly the extremities (ankles, wrists, phalanges) but also the large joints (Robinson, 1955; Lam et al.,2001; Hochedez et al.,2006; Quatresous, 2006; Saxena et al.,2006). Skin involvement is present in about 40-50% of cases, and consists of a pru-riginous maculopapular rash predominating on
the thorax. The clinical presentation may also in-volve facial oedema and, in children, a bullous rash with pronounced sloughing, localised pe-techiae and gingivorrhagia (Fourie and Morrison, 1979; Brighton et al.,1983). Radiological findings are normal, and biological markers of inflamma-tion (erythrocyte sedimentainflamma-tion rate and C-reac-tive protein) are normal or moderately elevated (Fourie and Morrison, 1979; Kennedy et al., 1980). Iridocyclitis and retinitis are the most common ocular manifestations associated with CHIKF; less frequent ocular lesions include episcleritis. All oc-ular manifestations have a benign course with complete resolution and preservation of vision. Retinitis shows gradual resolution over a period of 6 to 8 weeks (Mahendradas et al., 2008). Erratic, relapsing, and incapacitating arthralgia is the hall-mark of Chikungunya, although it rarely affects children. These manifestations are normally mi-gratory and involve the small joints of hands, wrists, ankles, and feet with pain on movement. The symptoms generally resolve within 7-10 days, except for joint stiffness and pain: up to 12% of patients still have chronic arthralgia three years after onset of the illness. Arthralgia experienced by CHIKV patients closely resembles the symptoms induced by other viruses like RRV and BFV (et al., 2002; Jacups et al., 2008). Neurological complications such as meningo-en-cephalitis were reported in a few patients during the first Indian outbreak in 1973, and during the 2006 Indian outbreak (Chatterjee et al., 1965 a, b; Ravi, 2006). Moreover, during the 2006 Indian-Ocean outbreak, rare cases of Guillain-Barré syndrome associated with CHIKV infection have been described (Wielanek et al., 2007; Lebrun et al., 2009). The possible mechanisms underlying these processes remain unknown, even if it was found that mouse CNS tissues such as the cho-roid plexi could also be targets of CHIKV, len-ding more credence to the fact that CHIKV in-fections do affect CNS cells and tissues (Couderc
CHIKF epidemics was obtained in La Réunion, Mauritius, and India, by comparing expected and observed mortality data. In all cases, during the months when the epidemics were raging, the ob-served mortality significantly exceeded the ex-pected rate. In particular, in La Réunion the monthly crude death rates in February and March 2006 were respectively 34.4% and 25.2% higher than expected. This corresponded to 260 excess deaths (an increase of 18.4%) with a rough estimate of the case-fatality rate for CHIKF of ≈1/1,000 cases. The case-fatality rate calculated on increased crude death rates in Mauritius and Ahmedabad, India, is substantially higher than that calculated in La Réunion: approximately 4.5% (15,760 confirmed or suspected cases and 743 excess deaths) and 4.9% (60,777 confirmed or suspected cases and 2,944 excess deaths), re-spectively (Beesoon et al., 2008; Mavalankar et al.,
2008). These differences may be attributed to many factors (greater disease severity, preexisting patient conditions, different patient management, or coincident excess deaths from other causes) but may also be due to a different efficacy of the surveillance systems for CHIKF, that probably worked poorly in Mauritius and India, leading to underestimation of the total number of cases (Fusco et al.,2010). The possible link between CHIKV infection and multiorgan failure is still under investigation.
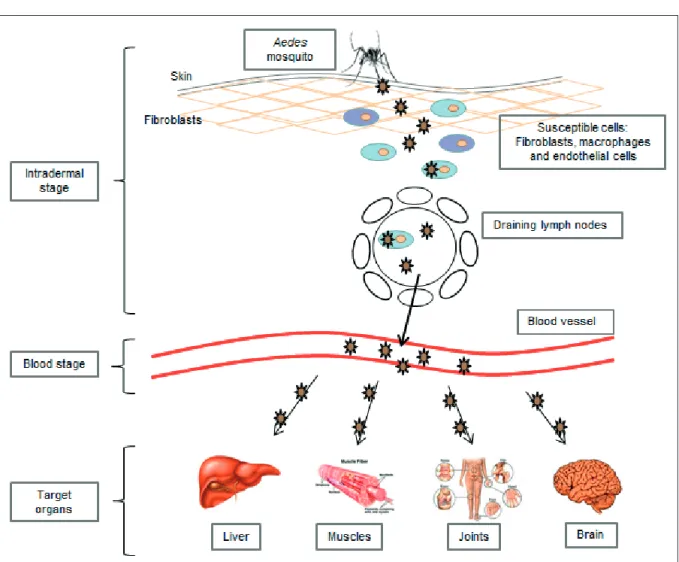
VIRUS DISSEMINATION AND TARGET ORGANS
Following intradermal inoculation by infected mosquitoes, CHIKV directly enters the subcuta-neous capillaries where its replication starts im-mediately (Figure 3) with some viruses infecting susceptible cells in the skin, such as macrophages or fibroblasts and endothelial cells. Local viral replication seems to be minor and limited in time, with the locally produced virus probably being transported to secondary lymphoid organs close to the site of inoculation, where infected migra-tory cells produce new viruses which can, in turn, infect susceptible resident cells. Even if the host is mounting a response to control the virus in the skin dermis, the virus disseminates quite rapidly to the blood circulatory system. Viruses produced in the draining lymph nodes are released into the
lymph circulation and then to the blood through the thoracic duct. Once in the blood, the virus will have access to various parts of the body, in-cluding the liver, muscle, joints and brain. In these tissues, the infection is associated with a marked infiltration of mononuclear cells, in-cluding macrophages, that can be considered Trojan horses for virus spread to sanctuary body sites. The pathological events associated with tis-sue infection are mostly subclinical in the liver (hepatocyte apoptosis) and lymphoid organs (adenopathy), whereas mononuclear cell infil-tration and viral replication in the muscles and joints are associated with very strong pain, with some patients presenting arthritis (Dupuis-Maguiraga et al.,2012).
During the first week of CHIKV infection, viremia can reach very high levels (viral loads of 3.3×109
copies/ml) (Parola et al.,2006). Thus, it remains unclear if the virus detected in the blood is re-leased from virus-infected peripheral blood mononuclear cells, or is spilled out from other replication sites.
IMMUNOPATHOGENESIS
The innate immune response is the first barrier against viruses, being able to inhibit viral repli-cation through cytolytic and non-cytolytic mech-anisms. The IFN system plays an important role in limiting virus spread at an early stage of in-fection.In vitrogrowth of all alphaviruses can be greatly suppressed by the antiviral effects of
IFN-α/βwhen it is added to cells prior to infection (Sourisseau et al., 2007; Courderc et al.,2008; Schilte et al.,2010). The finding that aberrant Type I interferon signalling in mice led to severe
forms of CHIKF (Couderc et al.,2008) further
highlighted the important roles cytokines play in the pathology of CHIKV infection.
Moreover, in a recent study Wauquier and col-leagues (2011) demonstrated that CHIKV infec-tion in humans elicits strong innate immunity in-volving the production of numerous proinflam-matory mediators. Interestingly, high levels of
Interferon IFN-α were consistently found.
T lymphocyte response in the early stages of the disease and a CD4+ T lymphocyte-mediated re-sponse in the later stages (Wauquier et al.,2011). CHIKV interactions with monocytes and with oth-er blood leukocytes induced a robust and rapid in-nate immune response with the production of spe-cific chemokines and cytokines, including IFN-α. The involvement of monocytes during the early phase of CHIKV infection in vivois massive, and infected monocyte/macrophages migrate in the synovial tissues of chronically CHIKV-infected pa-tients, where they contribute to the inflammation process. This may explain the persistence of joint symptoms despite the short duration of viremia
(Her et al., 2010). Infected monocyte/
macrophages may be the main cells responsible
for viral dissemination in other sanctuary body sites, such as the nervous system, and, in turn, may contribute to the development of clinical manifes-tations mediated by excess immune response. Usually, CHIKF is a self-limiting disease, with a defined duration of clinical course (7-10 days). Recovery is associated with a vigorous immune response, that may confer protection from re-in-fection. However, in some cases, chronic disease (arthralgia) may be established. Chronic symp-toms may persist even after clearance of the virus from the blood, but it is possible that an active viral reservoir persists locally in the joints. Five studies have tried to identify the factors associ-ated with chronic Chikungunya disease in groups of patients in Singapore (Chow et al., 2011), La
Réunion (Hoarau et al.,2010), Dakshina Kannada (India) (Manimunda et al.,2010; Chaaitanya et al.,2011), and Emilia Romagna (Italy) (Kelvin et al.,2011).
Regulatory mechanisms silencing the vigorous (even localized) inflammatory response seem to be required to prevent the establishment of chronic disease weeks or even months after viral clearance from the blood. The absence of such mechanisms leads to chronic arthralgia. In fact, in patients from the La Réunion study, various markers of inflammation (IFN-α, IL-6, monocyte chemotactic protein-1/CCL-2, IL-8, and matrix metalloproteinase 2) were detected in the syn-ovial fluid of a patient suffering from chronic pain, but not in patients who fully recovered (Hoarau et al.,2010). The persistence of a local reservoir of CHIKV in joints may therefore be characteristic of chronic disease, consistently with findings in the macaque model, in which CHIKV was detected after up to 90 days espe-cially in joint tissues, leading to chronic local in-flammation (Labadie et al., 2010). Moreover, Hoarauet al. (2010) reported high plasma
con-centrations of IL-12 and IFN-α mRNA in blood
mononuclear cells after the convalescent phase in patients with chronic disease between 6 months and 1 year after infection. In patients from Singapore, the concentrations of these two cytokines, measured by alternative techniques, peaked in the acute phase and returned to normal levels at 2-3 months, even in patients who still had clinical symptoms. According to these find-ings, Chaaithanya and colleagues (2011) and Kelvin and colleagues (2011) reported high lev-els of Th1-type cytokines in the blood of patients with chronic disease (Chaaitanya et al.,2011; Kelvin et al.,2011). Thus, despite certain dis-crepancies, the available studies suggest that chronic disease is associated with a de-regulation of inflammation during the acute and convales-cence phases. This lack of regulation results in a deleterious inflammatory process that persists for ≥ 1 year after the first clinical signs (Dupuis-Maguiraga et al.,2012).
Concerning the possible implication of viral fac-tors in the pathogenesis, attention has focused on the A226V mutation, that has been associated with enhanced replication and fitness of CHIKV in A. albopictusvector, and has also been shown to modulate the cholesterol requirement for
in-fection of insect cells (Tsetsarkin et al.,2007). A recent study by our group investigated the possi-ble involvement of A226V mutation in enhanc-ing human pathogenesis by testenhanc-ing the replica-tion competence in primate cell cultures of two isolates, differing for the presence or absence of this mutation (Bordi et al.,2011). We observed that the presence of A226V mutation did not in-fluence the replication kinetics on primate cells. Moreover, the two isolates displayed very similar time course of cytopathic effect onset, number and extent of CHIKV antigen-positive cells, as well as the t-shape of the virus-positive multicel-lular foci, thus suggesting a similar mechanism of spread of the virus in the infected cell cultures. In addition, we considered the possibility that the A226V mutation could be associated with partial resistance to the antiviral activity of recombinant IFN-αin classical experiments of virus replica-tion inhibireplica-tion. Surprisingly, the A226V-carrying strain was more susceptible than the wt virus to the antiviral action of IFN-α.
Overall, our result did not support the concept that A226V mutation confers a replicative ad-vantage in primate cell cultures, nor did it support the possibility that partial resistance to the in-hibitory action of IFN-αcould account for the ex-plosive spread of the mutated strain in the hu-man population in the countries where this mu-tation had occurred. However, the possibility that the interplay between the virus and the innate de-fence system may act at different levels of the virus/host interaction is to be taken into consid-eration, by exploring, for instance, other steps of the IFN response activation.
At the moment, understanding CHIKV immuno-biology is still in its infancy and there is a long way to go before answers related to the interac-tion between virus and host immunity are ob-tained. These will certainly be important in de-signing novel antiviral control strategies against the spread of CHIKV infection.
DIAGNOSIS
The case becomes probable if the patient has lived in or visited epidemic areas in a time frame con-sistent with the incubation period (WHO Guidelines for prevention and control of Chikungunya fever http://www.searo.who.int/ LinkFiles/Publication_SEA-CD-182.pdf (accessed Aug 01, 2011).
However, laboratory confirmation is crucial, be-cause the case should be distinguished from var-ious disorders with similar clinical manifesta-tions, such as dengue fever, other alphaviruses and arthritic diseases and also endemic malaria. The interpretation of laboratory findings is de-pendent on knowledge of the kinetics of viremia and antibody response in human beings. The de-tection of viral nucleic acid or of infectious virus in serum samples is useful during the initial viremic phase, at the onset of symptoms and nor-mally for the following 5-10 days, when CHIKV RNA reaches very high levels (viral loads of 3.3x109copies/ml) and can be easily detected.
Afterwards, the diagnosis is based mainly on the detection of specific immune response by sero-logical methods.
Molecular assays constitutes a rapid and sensi-tive technique for diagnosis of CHIKV infection during the early stages of illness before an anti-body response is evident. Conventional RT-PCR (Hasebe et al.,2002; Pfeffer et al.,2002) are avail-able, together with real time loop-mediated RT-PCR (Parida et al.,2007) and real time TaqMan RT-PCR assay targeting the envelope E1 gene (Pastorino et al.,2005) or the non-structural the nsP1 gene (Carletti et al.,2007). Moreover a one-step SYBR green-based real time assay targeting the non-structural nsp2 gene was described more recently (Ho et al.,2010).
Viral isolation can be performed from serum of infected patients on insect or mammalian cell lines (i.e. C6/36 or Vero E6) or by intracerebral in-oculation of 1-day-old mice during the early phase of the disease when the viral load is very high and the immune response is still not de-tectable. In fact, the presence of early antibody seems to prevent isolation of the virus, hence virus isolation has been shown to be successful largely in antibody-negative samples obtained on or before day 2 of illness (Panning et al.,2006). Moreover, viral isolation is useful for epidemiol-ogy or pathogenesis studies or for thorough mo-lecular characterization (Fusco et al.,2010).
The detection of CHIKV-specific immune re-sponse is based on serological methods such as enzyme-linked assays (ELISA), indirect im-munofluorescence assays (IFA), hemoagglutina-tion inhibihemoagglutina-tion (HI) and micro-neutralizahemoagglutina-tion (MNt).
IFA and ELISA are rapid and sensitive techniques for detection of CHIKV-specific antibodies, and can distinguish between IgG and IgM. IgM are detectable 2-3 days after the onset of symptoms and persist for several weeks, up to 3 months (Sam and AbuBakar, 2006; Litzba etet al.,2008). Rarely, IgM can be detected for longer periods, up to 1 year. CHIKV-specific IgG appear soon after IgM antibodies (2-3 days) and persist for years. Various in-house ELISA techniques using whole antigen or recombinant capsid or envelope anti-gens have been described (Cho et al., 2008). Commercial serological assays are available and results obtained from a comparison of the assays suggested that the sensitivity for detection of an early antibody response before day 5 is depend-ent on the strain of the virus used for the assay or the source of the antigen; assays based on re-combinant antigens might be too specific with regard to mutations (Cho et al.,2008; Litzba et al.,2008; Yap et al.,2010).
REFERENCES
AKAHATA W., YANG Z.Y., ANDERSEN H., SUN S.,
HOLDAWAYH.A., KONGW.P, LEWISM.G., HIGGSS., ROSSMANNM.G., SRINIVASR., NABELG.J. (2010). A VLP vaccine for epidemic Chikungunya virus pro-tects nonhuman primates against infection. Nat. Med.16, 334-338.
ARANKALLEV.A., SHRIVASTAVAS., CHERIANS., GUNJIKAR
R.S., WALIMBE A.M., JADHAV S.M., SUDEEP A.B.,
MISHRA A.C. (2007). Genetic divergence of
Chikungunya viruses in India (1963-2006) with spe-cial reference to the 2005-2006 explosive epidemic.
J. Gen. Virol.88, 1967-1976.
BEESOONS., FUNKHOUSERE., KOTEAN., SPIELMANA.,
ROBICHR.M. (2008). Chikungunya fever, Mauritius, 2006. Emerg. Infect. Dis.14, 337-338.
BLACKBURNN.K., BESSELAART.G., GIBSONG. (1995).
Antigenic relationship between chikungunya virus strains and o’nyong nyong virus using monoclonal antibodies. Res. Virol.146, 69-73.
BORDIL., CARLETTIF., CASTILLETTIC., CHIAPPINI R.,
SAMBRI V., CAVRINI F., IPPOLITO G., DI CAROA.,
CAPOBIANCHIM.R. (2008). Presence of the A226V mutation in autochthonous and imported Italian chikungunya virus strains. Clin. Infect. Dis.47, 428-429.
BORDIL., MESCHIS., SELLERIM., LALLEE., CASTILLETTI
C., CARLETTI F., CHIAPPINI R., DI CARO A., CAPOBIANCHIM.R. (2011). Chikungunya virus
iso-lates with/without A226V mutation show differ-ent sensitivity to IFN-α, but similar replication ki-netics in non human primate cells. New Microbiol.
34, 87-91.
BRIGHTON S.W., PROZESKYO.W., DE LA HARPE A.L.
(1983). Chikungunya virus infection. A retrospec-tive study of 107 cases. S. Afr. Med. J.63, 313-315. BRIGHTONS.W. (1984). Chloroquine phosphate
treat-ment of chronic Chikungunya arthritis: an open pi-lot study. S. Afr. Med. J.66, 217-218.
BRIOLANTS., GARIND., SCARAMOZZINON., JOUANA., CRANCE J.M. (2004). In vitro inhibition of
Chikungunya and Semliki Forest viruses replica-tion by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination.
Antiviral. Res.61, 111-117.
CARLETTI F., BORDI L., CHIAPPINI R., IPPOLITO G.,
SCIARRONEM.R., CAPOBIANCHIM.R., DICARO A., CASTILLETTIC. (2007). Rapid detection and quan-tification of Chikungunya virus by a one-step verse transcription polymerase chain reaction re-al-time assay. Am. J. Trop. Med. Hyg.77, 521-524. CHAAITANYAI.K., MURUGANANDAMN., SUNDARAMS.G.,
KAWALEKAR O., SUGUNANA.P., MANIMUNDA S.P.,
GHOSALS.R., MUTHUMANIK., VIJAYACHARIP. (2011).
Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral. Immunol.24, 265-271.
CHARRELR.N., DELAMBALLERIEX., RAOULTD. (2007). Chikungunya outbreaks - the globalization of vec-tor-borne diseases. N. Engl. J. Med.356, 769-771. CHARRELR., DELAMBALLERIEX. (2008). Chikungunya in
north-eastern Italy: a consequence of seasonal syn-chronicity. Euro Surveill.13, pii: 8003.
CHATTERJEES.N., CHAKRAVARTIS.K., MITRAA.C., SARKAR
J.K. (1965a). Virological investigation of cases with neurological complications during the outbreak of haemorrhagic fever in Calcutta. J. Indian Med. Assoc.45, 314-316.
CHATTERJEES.N., SARKARJ.K. (1965b). Electron
mi-croscopic studies of suckling mouse brain cells in-fected with Chikungunya virus. Indian J. Exp. Biol.
3, 227-234.
CHOB., KIMJ., CHOJ.E., JEONB.Y., PARKS. (2008).
Expression of the capsid protein of Chikungunya virus in a baculovirus for serodiagnosis of Chikungunya disease. J. Virol. Methods. 154, 154-159.
CHOWA., HERZ., ONGE.K., CHENJ.M., DIMATATACF., KWEKD.J., BARKHAMT., YANGH., RéNIAL., LEO
Y.S., NGL.F. (2011). Persistent arthralgia induced
by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J. Infect. Dis. 203, 149-57. COUDERC T., CHRéTIEN F., SCHILTE C., DISSON O.,
BRIGITTEM., GUIVEL-BENHASSINE F., TOURETY.,
BARAUG., CAYETN., SCHUFFENECKERI., DESPRèSP., ARENZANA-SEISDEDOSF., MICHAULTA., ALBERTM.L., LECUITM. (2008). A mouse model for Chikungunya:
young age and inefficient Type-I interferon sig-nalling are risk factors for severe disease. Plos Pathogens.4, e29.
COUDERCT., KHANDOUDIN., GRANDADAMM., VISSEC.,
GANGNEUX N., BAGOT S., PROST J.F., LECUIT M.
(2009). Prophylaxis and therapy for Chikungunya virus infection. J. Infect. Dis.200, 516-523. CUIF., RAYMONDM., QIAOC.L. (2006). Insecticide
re-sistance in vector mosquitoes in China. Pest. Manag. Sci.62, 1013-1022.
D’ORTENZIOE., GRANDADAMM., BALLEYDIERE., JAFFAR -BANDJEEM.C., MICHAULTA., BROTTETE., BAVILLE
M., FILLEUL L. (2011). A226V strains of
Chikungunya virus, Réunion Island, 2010. Emerg. Infect. Dis.17, 309-311.
DE LAMBELLERIE X., LEROY E., CHARREL R.N.,
TTSETSARKIN K., HIGGSS., GOULD E.A. (2008a).
Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come?
Virol. J.5, 33.
DELAMBALLERIEX., BOISSONV., REYNIERJ.C., ENAULTS.,
CHARRELR.N., FLAHAULTA., ROQUESP., LEGRANDR. (2008b). On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic. Dis.
8, 837-839.
an-tiviral therapeutics. Curr. Top. Microbiol. Immunol.
317,155-183.
DUPUIS-MAGUIRAGAL., NORETM., BRUNS., LEGRAND
R., GRASG., ROQUESP. (2012). Chikungunya
dis-ease: infection-associated markers from the acute to the chronic phase of arbovirus-induced arthral-gia. PLoS Negl. Trop. Dis.6, e1446.
ECKELSK.H., HARRISONV.R., HETRICKF.M. (1970).
Chikungunya virus vaccine prepared by Tween-ether extraction. Appl. Microbiol.19, 321-325. EDELMANR., TACKETC.O., WASSERMANS.S, BODISON
S.A., PERRYJ.G., MANGIAFICOJ.A. (2000). Phase II
safety and immunogenicity study of live Chikungunya virus vaccine TSI-GSD-218. Am. J. Trop. Med. Hyg.62, 681-685.
ENSERINKM. (2006). Infectious diseases. Massive
out-break draws fresh attention to little-known virus.
Science.311, 1085.
FOURIE E.D., MORRISON J.G. (1979). Rheumatoid
arthritic syndrome after Chikungunya fever. S. Afr. Med. J. 56, 130-132.
FUSCOF.M., PUROV., DICAROA., NICASTRIE., CARANNANTE
N., FAELLAF.S., BARZONL., DICESARES., PALùG.,
CAPOBIANCHI M.R., IPPOLITO G. (2006). Cases of
Chikungunya fever in Italy in travellers returning from the Indian Ocean and risk of introduction of the disease to Italy. Infez. Med.14, 238-245. FUSCOF.M., NICASTRIE., NISIIC., DICAROA, IPPOLITOG.
(2010). Chikungunya fever, a re-emerging disease.
Tropical and Emerging Infectious Diseases, Maltezou H.C. and Gikas A., 93-110, ISBN: 978-81-308-0389-0. HARRISON V.R., BINN L.N., RANDALL R. (1967).
Comparative immunogenicities of Chikungunya vaccines prepared in avian and mammalian tissues.
Am. J. Trop. Med. Hyg.16, 786-791.
HARRISONV.R, ECKELSK.H., BARTELLONIP.J. HAMPTON
C. (1971). Production and evaluation of a forma-lin-killed Chikungunya vaccine. J. Immunol. 107, 643-647.
HASEBEF., PARQUETM.C., PANDEYB.D., MATHENGEE.G.,
MORITAK., BALASUBRAMANIAMV., SAATZ., YUSOPA., SINNIAH M., NATKUNAM S., IGARASHI A. (2002). Combined detection and genotyping of Chikungunya virus by a specific reverse transcription-polymerase chain reaction. J. Med. Virol.67, 370-4.
HEARNH.J. JR., RAINEYC.T. (1963). Cross-protection in animals infected with Group A arboviruses. J. Immunol.90, 720-724.
HERZ., MALLERETB., CHANM., ONGE.K., WONGS.C., KWEKD.J., TOLOUH., LINR.T., TAMBYAHP.A., RéNIA
L., NGL.F. (2010). Active infection of human blood
monocytes by Chikungunya virus triggers an innate immune response. J. Immunol.184, 5903-5913. HOP.S., NGM.M., CHUJ.J. (2010). Establishment of
one-step SYBR green-based real time-PCR assay for rapid detection and quantification of Chikungunya virus infection. Virol. J.7, 13. HOARAUJ.J., JAFFARBANDJEEM.C., KREJBICHTROTOTP.,
DAS T., LI-PAT-YUEN G., DASSAB., DENIZOT M., GUICHARD E., RIBERA A., HENNI T., TALLET F., MOITONM.P., GAUZèREB.A., BRUNIQUETS., JAFFAR
BANDJEEZ., MORBIDELLIP., MARTIGNYG., JOLIVET
M., GAYF., GRANDADAMM., TOLOUH., VIEILLARDV., DEBRéP., AUTRANB., GASQUEP. (2010). Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol.184, 5914-5927.
HOCHEDEZP., JAUREGUIBERRYS., DEBRUYNEM., BOSSI
P., HAUSFATERP., BRUCKERG., BRICAIREF., CAUMES
E. (2006). Chikungunya infection in travelers.
Emerg. Infect. Dis.12, 1565-1567.
INOUE S., MORITA K., MATIAS R.R., TUPLANO J.V.,
RESUELLOR.R., CANDELARIOJ.R., CRUZD.J., MAPUA
C.A., HASEBEF., IGARASHIA., NATIVIDADF.F. (2003). Distribution of three arbovirus antibodies among monkeys (Macaca fascicularis) in the Philippines.
J. Med. Primatol.32, 89-94.
JACUPSS.P., WHELANP.I., CURRIEB.J. (2008). Ross River virus and Barmah Forest virus infections: a review of history, ecology, and predictive models, with im-plications for tropical northern Australia. Vector Borne Zoonotic. Dis.8, 283-297.
JAINM., RAIS., CHAKRAVARTIA. (2008). Chikungunya: a review. Trop. Doc.38, 70-72.
JOSSERANL., PAQUETC., ZEHGNOUNA., CAILLEREN., LE
TERTRE A., SOLET J.L., LEDRANS M. (2006). Chikungunya disease outbreak, Réunion Island.
Emerg. Infect. Dis.12, 1994-1995.
KAMY.W., ONGE.K., RéNIAL., TONGJ.C., NGL.F.
(2009). Immuno-biology of Chikungunya and im-plications for disease intervention. Microbes Infect.
11, 1186-1196.
KELVINA.A., BANNERD., SILVIG., MOROM.L., SPATARO
N., GAIBANIP., CAVRINIF., PIERROA., ROSSINIG., CAMERONM.J., BERMEJO-MARTINJ.F., PAQUETTES.G., XU L., DANESH A., FAROOQUI A., BORGHETTO I.,
KELVIN D.J., SAMBRI V., RUBINO S. (2011).
Inflammatory cytokine expression is associated with Chikungunya virus resolution and symptom severity. PLoS Negl. Trop. Dis.5, e1279.
KENNEDY A.C., FLEMING J., SOLOMON L. (1980).
Chikungunya viral arthropathy: a clinical descrip-tion. J. Rheumatol. 7, 231-236.
KENNEYJ.L., VOLKS.M., PANDYAJ., WANGE., LIANGX.,
WEAVERS.C. (2011). Stability of RNA virus
attenu-ation approaches. Vaccine.29, 2230-2234. KHANA.H., MORITAK., PARQUETMDMDELC., HASEBE
F., MATHENGEE.G., IGARASHIA. (2002). Complete
nucleotide sequence of Chikungunya virus and ev-idence for an internal polyadenylation site. Gen. Virol.83, 3075-3084.
KNUDSENA.B. (1995). Global distribution and
contin-uing spread of Aedes albopictus. Parassitologia.37, 91-97.
Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against Chikungunya virus. Vaccine.30, 6142-6149. LABADIE K., LARCHER T., JOUBERT C., MANNIOUI A.,
DELACHEB., BROCHARDP., GUIGANDL., DUBREILL., LEBONP., VERRIERB., DELAMBALLERIEX., SUHRBIER
A., CHERELY., LE GRAND R., ROQUES P. (2010).
Chikungunya disease in nonhuman primates in-volves long-term viral persistence in macrophages.
J. Clin. Invest.120, 894-906.
LAMS.K., CHUAK.B., HOOIP.S., RAHIMAHM.A., KUMARI
S., THARMARATNAMM., CHUAHS.K., SMITHD.W.,
SAMPSONI.A. (2001). Chikungunya infection-an emerging disease in Malaysia. Southeast Asian J. Trop. Med. Public Health32, 447-451.
LEBRUN G., CHADDA K., REBOUX A.H., MARTINETO.,
GAüZèREB.A. (2009). Guillain-Barré syndrome af-ter Chikungunya infection.Emerg. Infect. Dis.15, 495-496.
LEER.C., HAPUARACHCHIH.C., CHEN K.C., HUSSAIN
K.M., CHENH., LOWS.L., NGL.C., LINR., NGM.M., CHUJ.J. (2013). Mosquito cellular factors and func-tions in mediating the infectious entry of Chikungunya virus. PLoS Negl. Trop. Dis.7, e2050. LEMANTJ., BOISSONV., WINERA., THIBAULTL., ANDRéH., TIXIERF., LEMERCIERM., ANTOKE., CRESTAM.P., GRIVARD P., BESNARD M., ROLLOTO., FAVIER F.,
HUERREM., CAMPINOSJ.L., MICHAULTA. (2008).
Serious acute Chikungunya virus infection requir-ing intensive care durrequir-ing the Réunion Island out-break in 2005-2006. Crit. Care Med.36, 2536-2541. LEVITTN.H., RAMSBURGH.H., HASTYS.E., REPIKP.M.,
COLEF.E. JR., LUPTONH.W. (1986). Development of an attenuated strain of Chikungunya virus for use in vaccine production. Vaccine4, 157-162. LITZBAN., SCHUFFENECKERI., ZELLERH., DROSTENC.,
EMMERICHP., CHARRELR., KREHERP., NIEDRIGM. (2008). Evaluation of the first commercial Chikungunya virus indirect immunofluorescence test. J. Virol. Methods149, 175-179.
MAHALINGAMS., MEANGERJ., FOSTERP.S., LIDBURYB.A. (2002). The viral manipulation of the host cellular and immune environments to enhance propaga-tion and survival: a focus on RNA viruses. J. Leukoc. Biol.72, 429-439.
MAHENDRADASP., RANGANNAS.K., SHETTYR., BALUR., NARAYANAK.M., BABUR.B., SHETTYB.K. (2008).
Ocular manifestations associated with Chikungunya. Ophthalmol.115, 287-291.
MALLILANKARAMAN K., SHEDLOCK D.J., BAO H., KAWALEKAR O.U., FAGONE P., RAMANATHAN A.A.,
FERRAROB., STABENOWJ., VIJAYACHARIP., SUNDARAM
S.G., MURUGANANDAMN., SARANGANG., SRIKANTHP., KHANA.S., LEWISM.G., KIMJ.J., SARDESAIN.Y., MUTHUMANIK., WEINERDB. (2011). A DNA vaccine
against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and non-human primates. PLoS Negl. Trop Dis.5, e928.
MANIMUNDAS.P., VIJAYACHARIP., UPPOORR., SUGUNAN
A.P., SINGH S.S., RAI S.K., SUDEEP A.B., MURUGANANDAMN., CHAITANYA I.K., GURUPRASAD
D.R. (2010). Clinical progression of Chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by im-aging. Trans. R. Soc. Trop. Med. Hyg.104, 392-399. MAVALANKAR D., SHASTRI P., RAMAN P. (2007).
Chikungunya epidemic in India: a major public-health disaster. Lancet Infect. Dis.7, 306-307. MAVALANKARD., SHASTRIP., BANDYOPADHYAYT., PARMAR
J., RAMANIK.V. (2008). Increased mortality rate
as-sociated with Chikungunya epidemic, Ahmedabad, India. Emerg. Infect. Dis.14, 412-415.
MUTHUMANI K., LANKARAMAN K.M., LADDY D.J.,
SUNDARAMS.G., CHUNGC.W., SAKOE., WUL., KHAN
A., SARDESAIN., KIMJ.J., VIJAYACHARIP., WEINER
D.B. (2008). Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus.
Vaccine.26, 5128-5134.
PANNINGM., GRYWNAK., VANESBROECKM., EMMERICH
P., DROSTENC. (2008). Chikungunya fever in trav-elers returning to Europe from the Indian Ocean region, 2006. Emerg. Infect. Dis.14, 416-422. PARIDAM.M., SANTHOSHS.R., DASHP.K., TRIPATHIN.K.,
LAKSHMIV., MAMIDIN., SHRIVASTVAA., GUPTAN., SAXENAP., BABUJ.P., RAOP.V., MORITAK. (2007).
Rapid and real-time detection of Chikungunya virus by reverse transcription loop-mediated isothermal amplification assay. J. Clin. Microbiol.45, 351-357. PAROLAP., DELAMBALLERIEX., JOURDANJ., ROVERYC.,
VAILLANTV., MINODIERP., BROUQUIP., FLAHAULTA.,
RAOULT D., CHARREL R.N. (2006). Novel Chikungunya virus variant in travellers returning from Indian Ocean islands. Emerg. Infect. Dis.12, 1493-1499.
PASTORINOB., BESSAUDM., GRANDADAMM., MURRIS., TOLOUH.J., PEYREFITTEC.N. (2005). Development of a TaqMan RT-PCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. J. Virol. Methods 124, 65-71. PEDERSEN C.E, ROBINSON D.M., COLE F.E. (1972). Isolation of the vaccine strain of Venezuelan equine encephalomyelitis virus from mosquitoes in Louisiana. Am. J. Epidemiol.95, 490-496.
PEYREFITTEC.N., ROUSSETD., PASTORINOB.A., POUILLOT
R., BESSAUDM., TOCKF., MANSARAYH., MERLEO.L.,
PASCUALA.M., PAUPYC., VESSIEREA., IMBERTP.,
TCHENDJOUP., DURANDJ.P., TOLOUH.J., GRANDADAM
M. (2007). Chikungunya virus, Cameroon, 2006.
Emerg. Infect. Dis.13, 768-771.
PEYREFITTEC.N., BESSAUDM., PASTORINOB.A., GRAVIER
P., PLUMETS., MERLEO.L., MOLTINII., COPPINE., TOCKF., DARIESW., OLLIVIERL., PAGESF., MARTIN
R., BONIFACEF., TOLOUH.J., GRANDADAMM. (2008).
Circulation of Chikungunya virus in Gabon, 2006-2007. J. Med. Virol.80,430-433.
(2002). Specific detection of Chikungunya virus us-ing a RT-PCR/nested PCR combination. J. Vet. Med. B. Infect. Dis. Vet. Public Health.49, 49-54. PIALOUXG., GAüZèREB.A., JAURéGUIBERRYS., STROBEL
M. (2007). Chikungunya, an epidemic arbovirosis.
Lancet Infect. Dis.7, 319-327.
PILEJ.C., HENCHALE.A., CHRISTOPHERG.W., STEELE
K.E., PAVLINJ.A. (1999). Chikungunya in a North
American traveler. J. Travel. Med.6, 137-139. PISTONET., EZZEDINEK., SCHUFFENECKERI., RECEVEUR
M.C., MALVY D. (2009). An imported case of
Chikungunya fever from Madagascar: use of the sentinel traveller for detecting emerging arboviral infections in tropical and European countries.
Travel. Med. Infect. Dis.7, 52-54.
PLANTE K., WANG E., PARTIDOS C.D., WEGER J.,
GORCHAKOV R., TSETSARKIN K., BORLAND E.M., POWERS A.M., SEYMOUR R., STINCHCOMB D.T., OSORIOJ.E., FROLOVI., WEAVERS.C. (2011). Novel
Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism. PLoS Pathog.7, e1002142.
POLETTIP., MESSERIG., AJELLIM., VALLORANIR., RIZZO
C., MERLER S. (2011). Transmission potential of
chikungunya virus and control measures: the case of Italy. PLoS One6, e18860.
POWERSA.M., BRAULTA.C., TESHR.B., WEAVERSC.
(2000). Re-emergence of Chikungunya and o’ny-ong-nyong viruses: evidence for distinct geograph-ical lineages and distant evolutionary relationships.
J. Gen. Virol. 81, 471-479.
PROMED-MAIL. Chikungunya - Africa: Republic of the
Congo. ProMED-mail 2011; 13 Jun: 20110613.1806. <http://www.promedmail.org>.
PROMED-MAIL. Chikungunya - India (RA).
ProMED-mail 2012; 16 Jul: 20120716.1203694. <http://www. promedmail.org>.
PROMED-MAIL. Chikungunya and dengue - Cambodia. ProMED-mail 2012; 20 Sep: 20120920.1303166. <http://www.promedmail.org>.
PROMED-MAIL. Chikungunya: Papua New Guinea. ProMED-mail 2012; 10 Oct: 20121010.1335814. <http://www.promedmail.org>.
PROMED-MAIL. Chikungunya: Philippines (SA).
ProMED-mail 2013; 28 Jan: 20130128.1518853. <http://www.promedmail.org>.
PROMED-MAIL. Chikungunya: Papua New Guinea,
Australia ex Indonesia (Bali). ProMED-mail 2013; 20 Mar: 20130320.1594512. <http://www.promed-mail.org>.
QUATRESOUSI. (2006). E-alert 27 January: Chikungunya
outbreak in Réunion, a French overseas depart-ment. Euro Surveill.11, E060202.1.
RAVIV. (2006). Re-emergence of Chikungunya virus in India. Indian J. Med. Microbiol.24, 83-84.
RAVICHANDRANR., MANIANM. (2008). Ribavirin
thera-py for Chikungunya arthritis. J. Infect. Dev. Ctries.
2, 140-142.
REITERP., FONTENILLED. PAUPYC. (2006). Aedes al-bopictus as an epidemic vector of Chikungunya virus: another emerging problem? Lancet Infect. Dis.6, 463-464.
REZZAG., NICOLETTIL., ANGELINIR., ROMIR., FINARELLI
A.C., PANNINGM., CORDIOLIP., FORTUNAC., BOROS
S., MAGURANOF., SILVIG., ANGELINIP., DOTTORIM.,
CIUFOLINIM.G., MAJORIG.C., CASSONEA.; CHIKV STUDY GROUP. (2007). Infection with Chikungunya virus in Italy: an outbreak in a temperate region.
Lancet370, 1840-1846.
ROBINSONM.C. (1955). An epidemic of virus disease in
Southern Province, Tanganyika Territory, in 1952-1953. I. Clinical features. Trans. Royal Society Trop. Med. Hyg.49, 28-32.
RULLIN.E., GUGLIELMOTTIA., MANGANOG., ROLPHM.S.,
APICELLAC., ZAIDA., SUHRBIERA., MAHALINGAMS. (2009). Amelioration of alphavirus-induced arthri-tis and myosiarthri-tis in a mouse model by treatment with bindarit, an inhibitor of monocyte chemotac-tic proteins. Arthritis Rheum.60, 2513-2523. SAMI.C., ABUBAKARS. (2006). Chikungunya virus
in-fection. Med. J. Malaysia61, 264-269.
SAXENAS., SINGHM., MISHRAN., LAKSHMIV. (2006).
Resurgence of Chikungunya virus in India: an emerging threat. Euro Surveill.11, E060810.2. SCHILTEC., COUDERCT., CHRETIENF., SOURISSEAUM.,
GANGNEUXN., GUIVEL-BENHASSINEF., KRAXNERA.,
TSCHOPP J., HIGGS S., MICHAULT A., ARENZANA -SEISDEDOSF., COLONNAM., PEDUTOL., SCHWARTZO., LECUITM., ALBERTM.L. (2010). Type I IFN controls
Chikungunya virus via its action on non-hematopoietic cells. J. Exp. Med. 207, 429-442. SCHUFFENECKERI., ITEMANI., MICHAULTA., MURRIS.,
FRANGEULL., VANEYM.C., LAVENIRR., PARDIGONN.,
REYNES J.M., PETTINELLI F., BISCORNET L.,
DIANCOURTL., MICHELS., DUQUERROYS., GUIGONG., FRENKIELM.P., BRéHINA.C., CUBITON., DESPRèSP., KUNSTF., REYF.A., ZELLERH., BRISSES. (2006).
Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. Plos Medicine
3, 1058-1070.
SHARMAA., RAVIVY., PURIA., VIARDM., BLUMENTHAL
R., MAHESHWARIR.K. (2007). Complete inactivation
of Venezuelan equine encephalitis virus by 1,5-iodonaphthylazide. Biochem. Biophys. Res. Commun. 358, 392-398.
SHARMA A., GUPTA P., MAHESHWARI R.K. (2012).
Inactivation of Chikungunya virus by 1,5 iodon-apthyl azide. Virol. J.9, 301.
SOURISSEAUM., SCHILTEC., CASARTELLIN., TROUILLETC.,
GUIVEL-BENHASSINEF., RUDNICKAD., SOL-FOULONN.,
LEROUXK., PREVOSTM.C., FSIHIH., FRENKIELM.P., BLANCHETF., AFONSOP.V., CECCALDIP.E., OZDENS., GESSAIN A., SCHUFFENECKER I., VERHASSELT B.,
ZAMBORLINI A., SAïB A., REY F.A., ARENZANA
Chikungunya virus. PLoS Pathogen.3, e89. STRAUSSJ.H., STRAUSSE.M. (1994). The alphaviruses:
gene expression, replication and evolution.
Microbiol. Rev.58, 491-562.
TAUBITZ W., CRAMER J.P., KAPAUN A., PFEFFER M., DROSTENC., DOBLERG., BURCHARDG.D., LöSCHER
T. (2007). Chikungunya fever in travelers: clinical presentation and course. Clin. Infect. Dis.45, e1-4. TSETSARKINK.A., VANLANDINGHAMD.L., MCGEEC.E., HIGGSS. (2007). A single mutation in Chikungunya virus affects vector specificity and epidemic poten-tial. PLoS Pathog.3, 1895-1906.
VAZEILLEM., MOUTAILLERS., COUDRIERD., ROUSSEAUX
C., KHUN H., HUERREM., THIRIAJ., DEHECQJ.S., FONTENILLE D., SCHUFFENECKER I., DESPRES P.,
FAILLOUXA.B. (2007). Two Chikungunya isolates
from the outbreak of La Réunion (Indian Ocean) exhibit different patterns of infection in the mos-quito, aedes albopictus. PLoS One2, 1-9.
VOLKS.M., CHEN R., TSETSARKINK.A., ADAMSA.P.,
GARCIAT.I., SALLA.A., NASARF., SCHUHA.J., HOLMES
E.C., HIGGSS., MAHARAJP.D., BRAULTA.C., WEAVER
S.C. (2010). Genome-scale phylogenetic analyses of Chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates.
J. Virol.84, 6497-6504.
WANGE., VOLKOVAE., ADAMSA.P., FORRESTERN., XIAO
S.Y., FROLOVI., WEAVERS.C. (2008). Chimeric
al-phavirus vaccine candidates for Chikungunya.
Vaccine26, 5030-5039.
WANG D., SUHRBIER A., PENN-NICHOLSON A.,
WORARATANADHARMJ., GARDNERJ., LUOM., LET.T.,
ANRAKUI., SAKALIANM., EINFELDD., DONGJ.Y. (2011). A complex adenovirus vaccine against Chikungunya virus provides complete protection against viremia and arthritis. Vaccine. 29, 2803-2809.
WANGE., KIMD.Y., WEAVERS.C., FROLOVI. (2011). Chimeric Chikungunya viruses are nonpathogenic
in highly sensitive mouse models, but efficiently in-duce a protective immune response.J. Virol. 85, 9249-9252.
WAUQUIERN., BECQUARTP., NKOGHED., PADILLAC.,
NDJOYI-MBIGUINOA., LEROYE.M. (2011). The acute phase of chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J. Infect. Dis.204, 115-123. WEAVERS.C., OSORIOJ.E., LIVENGOODJ.A., CHENR.,
STINCHCOMBD.T. (2012). Chikungunya virus and prospects for a vaccine. Expert Rev. Vaccines11, 1087-1101.
WHITE A., BERMAN S., LOWENTHAL J.P. (1972). Comparative immunogenicities of Chikungunya vaccines propagated in monkey kidney monolay-ers and chick embryo suspension cultures. Appl. Microbiol.23, 951-952.
WIELANEKA.C., MONREDONJ.D., AMRANIM.E., ROGER
J.C., SERVEAUXJ.P. (2007). Guillain-Barré syndrome
complicating a Chikungunya virus infection.
Neurology69, 2105-2107.
WINTACHAIP., WIKANN., KUADKITKANA., JAIMIPUKT., UBOL S., PULMANAUSAHAKULR., AUEWARAKUL P.,
KASINRERK W., WENG W.Y., PANYASRIVANIT M.,
PAEMANEEA., KITTISENACHAIS., ROYTRAKULS., SMITH
D.R. (2012). Identification of prohibitin as a Chikungunya virus receptor protein. J. Med. Virol.
84, 1757-1770.
WOLFEN.D., KILBOURNA.M., KARESHW.B., RAHMAN
H.A., BOSIE.J., CROPPB.C., ANDAUM., SPIELMANA., GUBLERD.J. (2001). Sylvatic transmission of
ar-boviruses among Bornean orangutans. Am. J. Trop. Med. Hyg.64, 310-316.
YAPG., POKK.Y., LAIY.L., HAPUARACHCHIH.C., CHOW
A., LEOY.S., TANL.K., NGL.C. (2010). Evaluation