UNIVERSIDAD DE VALLADOLID
ESCUELA DE INGENIERIAS INDUSTRIALES
MÁSTER EN INGENIERÍA QUÍMICA
Recrystallization kinetics of API/polymer systems
Autor: Rodríguez del Egido, Miguel Guillermo
Responsable Intercambio UVa:
Mato Chaín, Rafael B.
Technische Universität Dortmund
TFM REALIZADO EN PROGRAMA DE INTERCAMBIO
TÍTULO: Recrystallization kinetics of API/polymer systems ALUMNO: Miguel Guillermo Rodríguez del Egido
FECHA: 18/04/2017
Palabras clave: Recristalización, compuesto farmacéutico, nifedipina, dispersión sólida, Avrami.
Resumen: El compuesto nifedipina es prácticamente insoluble en agua y consecuentemente no se alcanza todo el potencial de sus propiedades. Para superar estas limitaciones, se utiliza la técnica de dispersión sólida. Sin embargo, formulaciones en estado amorfo obtenidas tienden a recristalizar.
Se estudiaron diferentes factores influyentes en la recristalización. De acuerdo a la composición de la formulación, cuanta menor concentración del compuesto farmacéutico, mayor es el tiempo para cristalizar. Respecto a la humedad relativa (RH), existe una gran diferencia entre los valores iniciales y finales de agua absorbida en los experimentos con las RH implementadas. Respecto a la temperatura, cuanto menor es la temperatura, mayor es tiempo necesario para alcanzar el equilibrio. También se llevaron a cabo experimentos cambiando el polímero y el modo de preparar la formulación.
Para caracterizar las muestras se utilizaron Powder X-Ray Diffraction (PXRD), Differential Scanning Calorimetry (DSC) y espectrofotómetro.
Technische Universität Dortmund
Fakultät Bio- und Chemieingenieurwesen
Lehrstuhl für Thermodynamik
Recrystallization kinetics of API/polymer systems
Master-Thesis
Miguel Guillermo Rodríguez del Egido
Matriculation Number: 197306
Dortmund, 18.04.2017
Supervisor:
First Examiner:
M. Sc. Christian Lübbert
Prof. Dr. Gabriele Sadowski
i
Declaration of authorship
I affirm that this thesis is completely my work and that any material from other sources is
marked in the text to the best of my knowledge. I also assure that I have not submitted any
part of this work for a degree at any other institution.
Dortmund 18.04.2017
ii
Abstract
The goal of this master project is to study the crystallization of nifedipine from water content
absorbed by analyzing different influencing: active pharmaceutical ingredients (API) weight
fraction, temperature, relative humidity (RH), polymer and the method to prepare the
formulation.
The drug is practically insoluble in water, consequently its properties do not reach its full
potential. To overcome these limitations, the solid dispersion method was the solution. It was
originally used to improve the dissolution properties and the bioavailability of poorly water
soluble drugs by dispersing them into water soluble carriers.
The amorphous solid dispersions were prepared by spray drying method with a Büchi Mini
Spray Dryer B-290.
When preparing the formulation, the same steps were always followed in order to have the
same thermodynamic history for all the samples. When preparing the amorphous solid
dispersion, acetone was used as solvent. The inlet temperature was 60 °C and the outlet
temperature was in a range of 35-40 °C.
After spray drying or melting method, the results show that the solid dispersions were totally
amorphous. In order to characterize the solid state of the sample, powder X-ray diffraction
(PXDR) and differential scanning calorimetry (DSC) were used.
The Avrami equation has proved its validity to model the behavior followed by the
crystallization. The parameters obtained agree with the results achieved from the suspension
balance experiments. It has been shown that the assumption of n parameter equal to 4, which
is crystal growth in 3 dimensions, is correct.
According to the API weight fraction, the lower API concentration the sample contains, the
longer it takes to crystallize. It could be the case that with that weight fraction does not reach
the recommended dose, in that case it would have to reach a compromise between the weight
fraction and the improved properties achieved and the recommended dose. On the other side,
iii
In terms of relative humidity, a huge difference occurs among the several relative humidities
that have been implemented. These differences translate into variations in the time required to
reach the equilibrium and crystallize and the initial and final water content.
Regarding to the temperature, the lower temperature under the sample is at, the longer it takes
to crystallize. Thereby, in order to avoid crystallization, it is necessary to operate under a
minimum temperature.
In respect of the method to prepare the formulation, differences in time to crystallize emerged.
Thus, the time needed for the melting method, which is normally used in industry, is less than
the time required for spray drying method. In this manner, in order to avoid crystallization, it
might be considered a way to use and optimize the spray drying method on a large scale.
Respecting the polymer used in the formulation, it was noticed that PVAC retarded the time
to crystallize so that it better fulfills the purpose of avoiding crystallization than Resomer
R203s. In further investigations, more polymers will have to be studied in order to find the
iv
List of symbols
Symbol Interpretation
wAPI Weight fraction of API
wNIF Weight fraction of Nifedipine
Tin Inlet temperature
Tout Outlet temperature
List of abbreviations
Abbreviation Interpretation
API Active Pharmaceutical Ingredient
NIF Nifedipine
FDA Food and Drug Administration
RH Relative humidity
PXRD Powder X-Ray Diffraction
DSC Differential Scanning Calorimetry
UV-Vis Ultra Violet – Visible
A Absorbance
VLE Vapor Liquid Equilibrium
SVLE Solid Vapor Liquid Equilibrium
PVAC Polyvinyl acetate of molecular weight 90.000
g/mol
PVAC350 Polyvinyl acetate of molecular weight
350.000 g/mol
v
List of figures
Figure 1. Scheme of spray drying method [6] ...5
Figure 2 Hot melt extrusion process [7] ...7
Figure 3. PXDR pattern of an amorphous sample (wNIF 70%)...8
Figure 4. PXDR pattern of a recrystallized sample (wNIF 80%, PVAC350, 40°C, 75%RH) obtained after the suspension balance experiment ...8
Figure 5. DSC thermogram of NIF/PVAC (wNIF 70%, PVAC, 40°C, 75%RH) ...9
Figure 6. Behavior of solubility against temperature and API weight fraction [10] ... 10
Figure 7. Behavior of solubility and present phases against temperature and weight fraction [10] .... 11
Figure 8. Evolution of water weight fraction: (a) initial curve for amorphous formulation at temperature I, (b) at temperature II, and (c) at temperature III [10] ... Figure 9. Behavior of NIF solubility [10] ... 12
Figure 10. Behavior of water content and phases during crystallization for a wNIF 70% [10] ... 13
Figure 11. Interaction of the three components of the system ... 16
Figure 12. Interaction of the forces involved in the EoS... 17
Figure 13. Characteristic sigmoidal curve followed by crystallization [16] ... 20
Figure 14. Graph where the slope is the n parameter [17] ... 21
Figure 15. Molecular structure of NIF ... 22
Figure 16. Molecular structure of NIF polymorphs [23] ... 23
Figure 17. Behavior of the two polymorphs on heating and cooling [23] ... 24
Figure 18. Theoretical NIF pattern by PXRD ... 24
Figure 19. Büchi Mini Spray Dryer B-290 experimental installation ... 26
Figure 20. Suspension balance experimental installation ... 27
Figure 21. Rigaku Miniflex 600 equipment ... 29
Figure 22. TA Instruments DSC Q-2000 equipment ... 30
Figure 23. Eppendorf BioSpectrometer equipment ... 31
Figure 24. Fitting of the experimental and VLE NIF curves [25] ... 33
Figure 25. Sorption diagram for sample wNIF 80%, 40°C, 60°RH ... 34
Figure 26. Sorption diagram for sample wNIF 80%, 40°C, 75°RH ... 35
Figure 27. Sorption diagram for sample wNIF 80%, 40°C, 90°RH ... 35
Figure 28. Sorption diagram for sample wNIF 80%, 35°C, 75°RH ... 36
Figure 29. Sorption diagram for sample wNIF 80%, 30°C, 75°RH ... 36
Figure 30. Overlap of the two independent water sorption measurements obtained for wNIF=0.80 at 40 °C, 75% RH to check the reproducibility of the experiments ... 37
Figure 31. Evolution of water content along the weight fraction ... 38
Figure 32. Evolution of water content along RH... 39
Figure 33. Evolution of water content along temperature for wNIF 90% and 75%RH... 40
Figure 34. Evolution of water content along temperature for wNIF 80% and 75%RH... 40
Figure 35. Evolution of water content along temperature for wNIF 70% and 75%RH... 41
Figure 36. Evolution of water content along polymer for wNIF 80%, 40°C and 75%RH ... 42
Figure 37. Evolution of water content along the method to prepare the formulation for wNIF 80%, 40°C and 75%RH ... 43
vi
Figure 39. Evolution of concentration of crystals, water and API for reference sample (wNIF 80%,
40°C, 75%RH) ... 45
Figure 40. PXRD pattern of amorphous state of sample wNIF 80% ... 46
Figure 41. PXDR pattern of crystalline state of sample wNIF 80%, 40ºC and 75%RH ... 46
Figure 42. Coupling of the experimental curve and Avrami theoretical curve ... 49
Figure 43. K coefficient behavior with API weight fraction ... 50
Figure 44. K coefficient behavior with relative humidity... 51
Figure 45. K coefficient behavior as function of temperature... 53
Figure 46. Concentration of components in the liquid phase for sample wNIF 0.90, 40°C, 75%RH ... 58
Figure 47. Evolution of concentration of crystals, water and API for sample wNIF 0.90, 40°C, 75%RH ... 58
Figure 48. Concentration of components in the liquid phase for sample wNIF 0.70, 40°C, 75%RH ... 58
Figure 49. Evolution of concentration of crystals, water and API for sample wNIF 0.70, 40°C, 75%RH ... 59
Figure 50. Concentration of components in the liquid phase for sample wNIF 0.85, 40°C, 75%RH ... 59
Figure 51. Evolution of concentration of crystals, water and API for sample wNIF 0.85, 40°C, 75%RH ... 59
Figure 52. Concentration of components in the liquid phase for sample wNIF 0.75, 40°C, 75%RH ... 60
Figure 53. Evolution of concentration of crystals, water and API for sample wNIF 0.75, 40°C, 75%RH ... 60
Figure 54. Concentration of components in the liquid phase for sample wNIF 0.80, 40°C, 75%RH by melting-cooling ... 60
Figure 55. Evolution of concentration of crystals, water and API for sample wNIF 0.80, 40°C, 75%RH by melting-cooling ... 61
Figure 56. Concentration of components in the liquid phase for sample wNIF 0.80, 40°C, 90%RH ... 61
Figure 57. Evolution of concentration of crystals, water and API for sample wNIF 0.80, 40°C, 90%RH ... 61
Figure 58. Concentration of components in the liquid phase for sample wNIF 0.80, 40°C, 60%RH ... 62
Figure 59. Evolution of concentration of crystals, water and API for sample wNIF 0.80, 40°C, 60%RH ... 62
Figure 60. Concentration of components in the liquid phase for sample wNIF 0.90, 30°C, 75%RH ... 62
Figure 61. Evolution of concentration of crystals, water and API for sample wNIF 0.90, 30°C, 75%RH ... 63
Figure 62. Concentration of components in the liquid phase for sample wNIF 0.90, 35°C, 75%RH ... 63
Figure 63. Evolution of concentration of crystals, water and API for sample wNIF 0.90, 35°C, 75%RH ... 63
Figure 64. Concentration of components in the liquid phase for sample wNIF 0.80, 35°C, 75%RH ... 64
Figure 65. Evolution of concentration of crystals, water and API for sample wNIF 0.80, 35°C, 75%RH ... 64
Figure 66. Concentration of components in the liquid phase for sample wNIF 0.80, 30°C, 75%RH ... 64
Figure 67. Evolution of concentration of crystals, water and API for sample wNIF 0.80, 30°C, 75%RH ... 65
Figure 68. Concentration of components in the liquid phase for sample wNIF 0.70, 35°C, 75%RH ... 65
vii
Figure 70. Concentration of components in the liquid phase for sample wNIF 0.90 in Resomer R203s,
40°C, 75%RH ... 66
Figure 71. Evolution of concentration of crystals, water and API for sample wNIF 0.90 in Resomer R203s, 40°C, 75%RH ... 66
Figure 72. Concentration of components in the liquid phase for sample wNIF 0.80 in Resomer R203s, 40°C, 75%RH ... 66
Figure 73. Evolution of concentration of crystals, water and API for sample wNIF 0.80 in Resomer R203s, 40°C, 75%RH ... 67
Figure 74. Concentration of components in the liquid phase for sample wNIF 0.70 in Resomer R203s, 40°C, 75%RH ... 67
Figure 75. Evolution of concentration of crystals, water and API for sample wNIF 0.70 in Resomer R203s, 40°C, 75%RH ... 67
Figure 76. Calibration curve of NIF at 276 nm in acetonitrile ... 71
List of tables
Table 1. Thermal properties of the different polymorphs [24] ... 23Table 2. Conditions of the experiments in suspension balance ... 28
Table 3. PC-SAFT API parameter ... 32
Table 4. Binary interaction parameters ... 33
Table 5. Degree of crystallinity of the samples by mass balances ... 44
Table 6. Melting enthalpies obtained by DSC measurements ... 47
Table 7. Results from UV-Vis measurements ... 48
Table 8. K coefficients with API weight fraction variation ... 50
Table 9. K coefficients with relative humidity variation ... 51
Table 10. K coefficients with temperature variation for wNIF 90% ... 52
Table 11. K coefficients with temperature variation for wNIF 80% ... 52
Table 12. K coefficients with temperature variation for wNIF 70% ... 52
Table 13. K coefficients with polymer variation ... 53
Table 14. K coefficients with method of preparation variation ... 54
Table 15. Measurements in the spray drying process ... 57
Table 16. DSC method ... 69
Table 17. Data about samples and their DSC measurements ... 68
viii
Contents
Declaration of authorship ...i
Abstract ... ii
List of symbols ... iv
List of abbreviations ... iv
List of figures ... v
List of tables ... vii
1. Aim of the project ...1
2. Theory ...3
2.1 Stabilization of APIs in polymeric matrices (solid dispersions) ...3
2.1.1 Methods of preparation ...4
2.1.2 Solid state characterization ...7
2.2 Phase behavior of API/polymer-formulations ...9
2.3 Influence of humidity on the phase behavior ... 13
2.4 Estimation of crystallinity via mass balances and water sorption ... 14
2.5 Calculation of activity coefficients via PC-SAFT ... 16
2.6 JMAK equation ... 19
3. Materials and experimental methods ... 22
3.1 Materials ... 22
3.2 Experimental methods ... 25
3.2.1 Preparation of formulations via spray drying ... 25
3.2.2 Suspension balance experiments ... 26
3.2.3 Verification of crystallinity via PXRD and DSC ... 28
3.2.5 UV-Vis measurements ... 30
4. Results and discussion... 32
4.1 Modeling the phase behavior of API/polymer-formulations ... 32
4.2 Predicting the influence of humidity on the phase behavior ... 34
4.3 Results of suspension balance experiments ... 36
4.3.1 Influence of wNIF... 37
4.3.2 Influence of RH ... 38
4.3.3 Influence of Temperature ... 39
4.3.4 Influence of polymer type ... 41
4.3.5 Influence of the method to prepare the formulation ... 42
ix
4.5 Results of UV-Vis measurements ... 48
4.6 Determination of Avrami-coefficients... 49
4.6.1 Influence of wNIF ... 50
4.6.2 Influence of RH ... 51
4.6.3 Influence of Temperature ... 52
4.6.4 Influence of polymer type ... 53
4.6.5 Influence of the method to prepare the formulation ... 54
5. Conclusion ... 55
1
1. Aim of the project
Nifedipine (NIF) is a calcium channel blocker, widely used clinically as a coronary
vasodilator. Pharmaceutical preparations are available as capsules, tablets, and solutions, and
the drug is usually administered orally or intravenously. The drug is practically insoluble in
water, and is reported to be highly light sensitive
The current way of administration implicates a number of weaknesses. In fact, NIF is usually
provided as solution using liquid oral drug preparation or by cutting a soft gelatin capsule and
by squeezing out the fluid content beneath the tongue [1]. This mode of administration can
lead to dosage and assimilation variability. The administration of NIF solid dispersions (SD),
by using a suitable polymeric carrier, comes out an interesting modus operandi in order to
progress into a better dose precision, residence time and patient compliance.
The SD method was initially used to ameliorate the dissolution properties and the
bioavailability of poorly water soluble drugs by dispersing them into water soluble carriers.
Dissolution deceleration through SD method using water insoluble polymer for the
development of controlled release dosage forms has become a field of interest in latest years
[2].
The progress of controlled release SD has a great advantage for avoiding the risk of a sudden
release of drug; as the structure of the solid dispersion allow a homogeneous dispersion of the
drug molecules [2].
Amorphous formulations have attracted considerable attention in the pharmaceutical field. As
the molecular structures of drug candidates become increasingly complex, solubility often
becomes a limiting factor in dissolution and oral absorption. The use of the amorphous form
is a promising strategy to battle these limitations due to its higher free energy and, therefore,
higher apparent solubility than its crystalline equivalent. However, such amorphous
formulations are metastable and tend to convert spontaneously to the crystalline state.
When crystallization occurs during processing, or during the shelf-life of the pharmaceutical
product, bioavailability and product efficacy may be adversely affected. Therefore, an
understanding of the crystallization kinetics from the amorphous state and the prediction of
2
Long term stability tests are imposed by Food and Drug Administration (FDA) at defined
Temperature and relative humidity (RH) conditions with which their long term stability is
verified and the appropriate polymer and concentration for the formulations are acquired.
The aim of this master thesis is to study the crystallization velocity of NIF formulations from
the water content absorbed analyzing different influencing factors: active pharmaceutical
ingredient (API) weight fraction, temperature, RH, polymer and the method to prepare the
formulation. It brings into focus the characterization of the ternary system
(API-polymer-water).
Finally, the predicted crystallization profiles are calculated and compared with the
3
2. Theory
An API is the chemical compound of any formulation which is pharmaceutically active. Some
formulations, such as integration treatment, have multiple active ingredients to treat different
diseases.
All drugs are made up of two core elements: the API, which is the central ingredient, and the
excipient, the substance inside the drug that helps deliver the medication to the desired place
within the organism. Excipients are inactive substances, such as polymers, and not chemically
active.
According to the U.S. Food and Drug Administration, an API is defined as:
“Any substance or mixture of substances intended to be used in the manufacture of a drug
product and that, when used in the production of a drug, becomes an active ingredient in the
drug product. Such substances are intended to furnish pharmacological activity or other direct
effect in the diagnosis, cure, mitigation, treatment or prevention of disease or to affect the
structure and function of the body.” [3].
2.1 Stabilization of APIs in polymeric matrices (solid dispersions)
Amorphous formulations have attracted considerable interest in the pharmaceutical field as a
way of improving bioavailability [2,4]. As the molecular structures of drug candidates
become increasingly complex, solubility often becomes a limiting factor in dissolution and
oral absorption.
The crystalline state is thermodynamically the most stable solid state, and is characterized by
both lower solubility and higher chemical stability [30].
When crystallization occurs during processing, or during the useful life of the pharmaceutical
product, bioavailability and product efficacy may be adversely affected.
The process of crystallization is known to be composed of two major steps: nucleation and
crystal growth, and the rates are generally governed by molecular mobility affecting the
diffusion rate of molecules and thermodynamic factors such as the Gibbs free energy and
4
Preparation of poorly water-soluble APIs into amorphous forms improves their solubility.
However, amorphous solids are often physically metastable because of their high energy state,
and crystallization during storage presents a problem.
A promising strategy to improve the dissolution limited bioavailability would be to use the
amorphous state and take advantage of the higher solubility of this high free energy state. On
the other hand, because amorphous drugs are in a higher free energy state than a
corresponding crystalline state, re-crystallization is thermodynamically favored.
The phase behavior of solid dispersions can be extremely complicated, whereby the API can
potentially be present in its crystalline state (which may involve one or more polymorphic
forms) or as a fully or partially amorphous form. For amorphous solid dispersions, one
important aspect of the phase behavior is the miscibility of the amorphous phases of the drug
and the polymeric carrier.
2.1.1 Methods of preparation
There are numerous methods to prepare a solid dispersion. Below it is proceed to describe
some of them [2]:
Spray drying
Spray drying is a unit operation capable of transforming solutions or suspensions into a solid
product. The spray drying technique comprises four basic steps: atomization of the liquid,
mixing of the liquid with the drying gas, evaporation of the liquid and separation of the dried
particles from the gas [2].
The liquid solution is transferred from the beaker to the nozzle entrance by a pump system. In
case of solid dispersion preparation, the used solvents are mostly organics.
As the slurry enters the tower, it is atomized into fine droplets. Partly because of the high
surface tension of water and partly because of the hydrophobic/hydrophilic interactions
5
Several types of atomization devices are available, depending on the type of energy that is
involved: centrifugal energy, pressure energy, kinetic energy and vibrations.
All spray dryers use some type of atomizer or spray nozzle to disperse the liquid or slurry into
a controlled drop size spray. The most common of these are rotary disk and single-fluid high
pressure swirl nozzles.
The small size of the drops (averaging 100 micrometers in diameter) results in a relatively
large surface area which dries quickly. As the water dries, the carrier forms a hardened shell
around the load. The final product is removed from the gas phase in a cyclone and collected in
a cyclone at the end of the process [5]
A general scheme for a spray drying equipment is depicted in the following Figure 1:
Figure 1. Scheme of spray drying method [6]
Melting method
The melting method involves melting of a physical mixture of drug and carrier to the liquid
6
one of the advantages is that it is a solvent free method. However, the API and the excipient
should be miscible and mixed well [4].
Melting solvent method
The melting solvent technique is a combination of the solvent evaporation method and
melting method [2].
It is carried out by the dissolution of the drug in an appropriate solvent and the mixture of this
solution with the molten carrier followed by cooling for solidification. The advantage of this
method is that it needs inferior temperatures with lesser risk of decomposition of thermolabile
drugs than other methods.
Solvent evaporation method
This technique consists of dissolving the drug and excipient simultaneously in a common
solvent, followed by the withdrawal of the solvent by evaporation.
Many drugs and carriers cannot be utilized for others methods, such as melting method, due to
their high melting points, then they can be used for solvent evaporation method [2].
Hot melt extrusion
The advantages of this technique include lower temperature than other methods and shorter
residence time of the drug carrier mixture. The physical mixture of drug and excipient is
moved into the heated chamber of extruder at a controlled rate.
Since the physical mixture is carried through heated screws, it is melted, which allows
homogeneous mixing. In the last step of the process the melt is shaped in the required form
7
The following Figure 2 shows a general outline of the process:
Figure 2 Hot melt extrusion process [7]
Supercritical antisolvent method
It is said a single fluid phase is supercritical when it is above its critical pressure and
temperature. Carbon dioxide is the most used supercritical fluid.
Several terms are used according to the technique by which supercritical fluid and solution are
mixed: (1) precipitation from supercritical solutions by fast expansion of supercritical
solution, (2) precipitation from saturated solution using supercritical fluid as an antisolvent,
(3) precipitation from gas saturated solutions [2].
2.1.2 Solid state characterization
Owing to the importance of the nature of solid dispersions, either crystalline or amorphous
state, it is needed to verify which state has been achieved both at the beginning and at the end
of each experiment.
For this reason, several techniques have been used. These are Powder X-Ray Diffraction
(PXRD) and Differential Scanning Calorimetry (DSC) since they are the most generally used
techniques to define the solid state. Spectrophotometer has been used in order to measure the
8
PXDR is a technique using X-Ray to illustrate the crystalline state of materials. The scattering
of X-rays from atoms produce a diffraction pattern that contains information about the atomic
structure. Amorphous materials do not produce any significant peak in diffraction pattern,
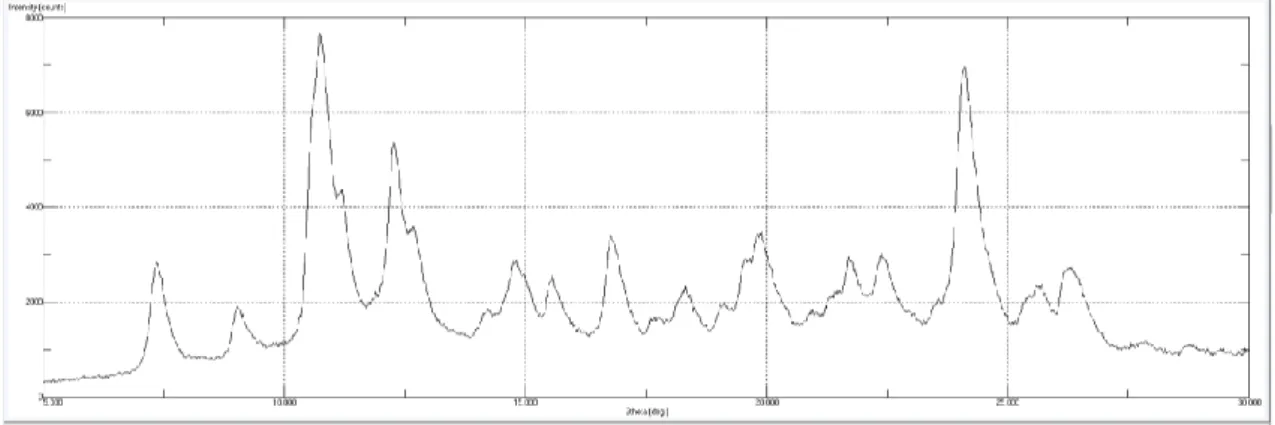
while crystalline materials are completely the opposite as it can be seen in Figures 3 and 4 [8].
Figure 3. PXDR pattern of an amorphous sample (wNIF 70%)
Figure 4. PXDR pattern of a recrystallized sample (wNIF 80%, PVAC350, 40°C, 75%RH) obtained
after the suspension balance experiment
DSC is an analytical technique in which the difference in heat flow between a sample and an
inert reference is measured as a function of time and temperature as both are subjected to a
controlled environment of time and temperature [9].
DSC analyses give information about melting temperature and enthalpy by the study of
9
Figure 5. DSC thermogram of NIF/PVAC (wNIF 70%, PVAC, 40°C, 75%RH)
A UV-VIS Spectrophotometer has been used to measure the absorbance at a particular
wavelenght in order to verify the composition of both components at the end of experiment.
2.2 Phase behavior of API/polymer-formulations
In this section, it will be discussed the phase behavior of API-polymer formulations. The
phase behavior of the system depends on the conditions in which the work is being done,
thereby different cases can be found.
The knowledge of the phase behavior is of great interest for practical application. When
recrystallization or an amorphous phase separation of the API / polymer system is observed,
the end of the shelf life of the formulation is achieved.
165.95°C 158.58°C 49.92J/g -0.8 -0.6 -0.4 -0.2 0.0 0.2 0.4 H e a t F lo w ( W /g )
-50 0 50 100 150 200 250
Temperature (°C) Sample: PVAc+NIF_wAPI70%_40_75%RH2
Size: 7.4000 mg
Method: Miguel DSC
File: \\...\PVAC+NIF_WAPI70%2.001 Operator: MR
Run Date: 31-Oct-2016 12:31
Instrument: DSC Q2000 V24.11 Build 124
10
The stability of the mixture is determined by the solubility. The maximum quantity of API
that can be dissolved in the polymer without crystallization is the API solubility in the
polymer and thus depends on the phase behavior of the resulting API/polymer formulation.
Figure 6. Behavior of solubility against temperature and API weight fraction [10]
As it can be seen in Figure 6, solubility separates two contrasting areas. Below the solubility
concentration the API-polymer formulation is stable and at API concentrations higher than the
solubility, the system tends to crystallize as it is supersaturated and unstable.
On the other hand, it also affects the miscibility gap between the compounds. Therefore it can
be distinguished several zones as it can be seen in Figure 7. The green area (upper area)
corresponds to the stable zone in which the system is in amorphous state. In the purple sector
(upper inner area of the miscibility curve), it is found two liquid phases corresponding to
polymer and water. In the dark orange sector (lower area) within the miscibility gap, it is
found two liquid phases as in the previous sector and recrystallization may occur as well.
Finally in the light orange area there is recrystallization as the concentration is greater than the
11
Figure 7. Behavior of solubility and present phases against temperature and weight fraction [10]
If a high API concentration is present, the solubility of the API in the polymer/water mixture
is exceeded, the API recrystallizes and a vapor-solid-liquid equilibrium ensues.
It is assumed that the vapor phase still consists exclusively of water. In the liquid phase
amorphous API and polymer as well as sorbed water are present. The solid phase consists of
pure crystalline API. The recrystallization of the API reduces the API concentration in the
12
The evolution of the water weight fraction from the amorphous to crystalline state can be
followed in the next Figure 8.
In the next Figure 9, it can be observed the evolution of solubility in this specific case of a
Nifedipine-PVAC sample in conditions of 40°C and 75% RH.
Figure 9. Behavior of NIF solubility [10]
Figure 8. Evolution of water weight fraction: (a) initial curve for amorphous formulation at
13 2.3 Influence of humidity on the phase behavior
In this chapter, it will be discussed the effect of humidity in the polymer-API system as it is
being studied the crystallization by the water absorbed. Amorphous solid dispersions are
prone to absorb water which drives to crystallization of API. Both the API solubility and
water sorption depends on type of API and polymer on the formulation, as well as the
temperature and the RH [10].
The water sorption of the formulations depends on the physical state of them. Phase
transformations can be observed as function of time.
Figure 10. Behavior of water content and phases during crystallization for a wNIF 70% [10]
As it can be seen in the previous Figure 10, the maximum value of water weight fraction
corresponds to amorphous nature and as time goes on the sample begins to crystallize until
reaching the minimum possible water value [11].
As the relative humidity of the amorphous system was increased, a decrease in the glass
transition temperature was noticed indicating an increase in molecular mobility which leads to
a faster crystallization [12]
Therefore, on the one hand, water absorbed from the vapor phase induces API crystallization;
and on the other hand, the crystallinity of the API/polymer system determines the quantity of
14 2.4 Estimation of crystallinity via mass balances and water sorption
In order to estimate the crystallinity of the sample from the water sorption, obtained from the
suspension balance experiments, it is necessary to make use of mass balances and
Vapor-Liquid-Equilibrium (VLE) and Solid-Vapor-Vapor-Liquid-Equilibrium (SVLE).
Regarding to the mass balances, it was required to implement a global mass balance to the
whole system and a mass balance to every substance taking into account the two existing
phases: solid and liquid.
One assumption taken is that the water is absorbed only by the amorphous liquid phase. The
solid phase consists exclusively of crystalline API, polymer and water are insoluble in the API
crystal.
Furthermore, the mass of the amorphous polymer in the liquid phase of the water-free system
is equal to the mass of polymer in the water-loaded system.
The mass of API in the saturated liquid phase and in the crystalline solid corresponds to the
mass of the API in the water-free system.
Global mass balance:
(1)
In this expression, mean the total liquid and solid phase respectively.
Water mass balance:
15
In which the total water mass is the sum of water mass in the liquid phase and the water mass
in the solid phase. This last one is equal to zero, , due to it was assumed that the solid
phase, that is the crystals, is completely composed by NIF.
On the other hand, the liquid water mass depends on the absorbed water weight fraction and
the formed crystal percentage per total mass .
(3)
Polymer mass balance:
(4)
Where the polymer total mass is equal to the sum of polymer liquid phase mass and the
polymer solid phase mass. As it was said before, as it was assumed that the solid phase is
completely composed by API, then the polymer mass in the solid phase is zero.
(5)
And the polymer liquid phase weight fraction depends on the API and H2O weight fraction.
(6)
API mass balance:
16
In this expression, the API total mass is equal to the addition of API liquid phase mass and
API solid phase mass. As it was assumed that the solid phase is completely composed by
nifedipine, then .
On the other side, the liquid phase weight fraction depends on the absorbed water and the API
water free-liquid phase weight fraction ( ), which was obtained from the VLE.
(8)
2.5 Calculation of activity coefficients via PC-SAFT
The Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) is an equation of state
designed for modeling mixtures of all types of substances: gases, solvents and polymers.
PC SAFT is satisfactory for the calculation of phase equilibrium and thermodynamic
properties of pure components and mixtures. It is found to be more precise comparing to other
equation of state (EoS) for correlation of experimental data and more predictive when applied
to mixtures. It is very reliable for extrapolations over the regions where parameters were
adjusted. Comparison to the original SAFT equation of state reveals significant improvement
for both, mixtures of small components and polymer solutions [13].
17
A huge advance in modeling phase behavior of polymer systems has been made during the
last years with use of EoS. In many such theories chain-like molecules are modeled as chains
of freely-jointed spherical segments.
The PC-SAFT equation of state is based on perturbation theory with the hard chain as
reference fluid. The perturbation contributions account for dispersive attractions as well as
association interactions and the reduced residual Helmholtz energy [13].
In accordance with this model, molecules are determined by three pure component
parameters:
- Attraction parameter ε
- Segment number m
- Segment diameter σ
In spite of its clarity, this molecular model considers the vital characteristics of real
molecules. Those characteristics are:
- Repulsive interactions
- Non-spherical shape of molecules (chain formation)
- Attractive interactions (dispersion)
18
The hard chain contribution represents the repulsions between the molecules. The attractions
of molecules are considered by dispersive interactions and strong hydrogen bonding which
exists between the associating molecules.
Therefore, the free residual energy ares is calculated [14]:
(9)
Despite considerable work on further developments, one of the most successful models
remains the original SAFT equation of state. However, in the dispersion term of the original
SAFT model, the chain-like structure of polymer compounds is not taken into account.
In the Perturbed-Chain SAFT equation of state, the dispersive forces are accounted for
applying a perturbation theory of second order (Barker and Henderson), using an expression
for the radial pair distribution function of a hard-chain reference fluid [13]
For utilization of the PC-SAFT equation of state to real substances, the integral in the
dispersion term is replaced by a power series in density, where the coefficients show a simple
dependence on segment number.
To characterize an associating substance, the number of association sites as well as their type
has to be assigned according to the molecular structure. Five pure component parameters for
associating substances have to be identified:
Segment number:
19
Segment diameter:
(11)
Dispersion energy parameter:
(12)
Association energy:
(13)
Association volume:
(14)
2.6 JMAK equation
In order to represent a model for the kinetics of crystallization, it has been used the Avrami
equation. It describes the transformation of one phase from another by the growth of nuclei
that are randomly formed in the primary phase.
The Avrami equation is also known as Johnson Mehl Avrami Kolmogorov (JMAK) equation
[15].
The crystallization is usually observed to follow a characteristic sigmoidal curve in which it
can be differentiated three zones. At the beginning, the crystallization rate is slow as it is the
time required for a significant number of nuclei of the new phase to form. The intermediate
20
again since most of the compound has been crystallize and there is less material to convert,
the particles already existing start to touch each other.
Figure 13. Characteristic sigmoidal curve followed by crystallization [16]
It has been assumed important simplifications to follow Avrami equation [17]:
- Nucleation occurs randomly and homogeneously over the entire untransformed
portion of the material.
- Growth rate does not depend on the degree of transformation.
- Growth happens at the same velocity in all directions, the growth is isotropic.
Thus, the number of nuclei is based on the volume, the nucleation rate and the time interval in
which it is measured.
(15)
Developing this initial equation, the final equation is reached, the usual form of Avrami
21
(16)
Where
It can also be written as:
(17)
This equation allows the estimation of the transformation constants, K and n, as it can be seen
in the next Figure 14.
Figure 14. Graph where the slope is the n parameter [17]
K and n are transformation parameters. The n parameter depends on the dimension of the
22
3. Materials and experimental methods
3.1 Materials
Nifedipine was purchased from Tokyo Chemical Industry CO., LTD. (Tokyo, Japan) with a
purity of >98.0%
Poly(vinyl acetate) (PVAC) of a molecular weight of 90.000 g/mol was acquired from
Polysciences Inc. (Warrington, PA, USA). Poly(vinyl acetate) (PVAC) of a molecular weight
of 350.000 g/mol was purchased from Scientific Polymer Products Inc. (Ontario, NY, USA).
Resomer® R203 S was acquired from Evonik Industries (Darmstadt, Germany).
Acetone with a purity of 99.9%, was purchased from VWR International GmbH (Darmstadt,
Germany) was used to prepare the formulations of API and the polymers for spray drying.
NIF is a yellow crystalline substance, practically insoluble in water but soluble in ethanol. It
has a molecular weight of 346.3 g/mol and a melting point of 173 °C.
NIF is an antianginal drug belonging to a class of pharmacological agents, the calcium
channel blockers. NIF is used to treat hypertension (high blood pressure) and angina (chest
pain). It works by relaxing the muscles of your heart and blood vessels. [19].
Nifedipine has the following structural formula [20]:
23
Polymorphism, that is the occurrence of chemical compounds characterized by the same
structural formula but different crystal forms, is a phenomenon of fundamental importance in
organic chemistry as different molecular structure may cause considerable variations in the
physical and chemical properties of the compound such as the melting temperature or
enthalpy. In the case of nifedipine, three polymorphs have been described: modification III,
modification II (or β) and modification I (or α). Only β and α polymorphs are metastable and
stable [21,22].
Figure 16. Molecular structure of NIF polymorphs [23]
Table 1. Thermal properties of the different polymorphs [24]
Properties α NIF β NIF
Melting temperature (°C) 169-173 162-164
Melting enthalpy (J/g) 104 71
In order to differentiate the two polymorphs, it was also found the different behavior on
heating and cooling. It can be appreciated, in Figure 17, the contract between the shape of the
24
Figure 17. Behavior of the two polymorphs on heating and cooling [23]
According to the X-Ray diffraction, Figure 18 is the pattern of the β polymorph [23], which
will be used later to distinguish them as well.
25 3.2 Experimental methods
In the experimental procedure, different stages can be distinguished:
3.2.1 Preparation of formulations via spray drying
The exact quantity of polymer and API contained in each sample was weighted by a balance
from Sartorius (Göttingen, Germany) following the selected rates of 70%, 75%, 80%, 85%,
90% NIF. Every sample was 1g.
Next, 100 mL of acetone were added to the mixture to keep a rate of 1/100. Before
performing the spray dying, it was needed to stir the solution in order to have it completely
dissolved and homogeneous.
In order to perform the solid dispersion preparation, a Mini Spray Dryer B-290 from Büchi
has been used.
Firstly, a nitrogen flow was fed into the equipment, so the oxygen inside decreases, to set up
inert conditions in the spray dryer. Regarding to aspiration, it was established at a value of
90% of the total capacity of the system.
Once the system was under inert conditions, the heater was started; the selected inlet
temperature was 60°C, with which 35-40 °C was reached as outlet temperature. After the
selected inlet temperature was achieved, the equipment was drained with pure acetone for 5
minutes to get the drying conditions. The pump rate was set at a point of 30% of its capacity.
Afterwards, the spray dryer method started when the pure solvent was switched to the
previously prepared solution. At the end of the process, it was switched back to acetone to
clean nozzle and tubes.
Finally, the powder resulting from the process was collected with a plastic spoon and stored in
26
Figure 19. Büchi Mini Spray Dryer B-290 experimental installation
3.2.2 Suspension balance experiments
The suspension balance is the equipment where the water sorption and recrystallization have
taken place. Once the solid dispersion was ready from spray drying, the sample was brought
here immediately.
Firstly, the solid dispersion was placed in a glass pan which went to the interior of the
chamber. Here, the working conditions were vacuum with respect to pressure and the selected
temperature during 2 hours, the time needed to reach equilibrium, in order to dry the sample.
Once the equilibrium is achieved, the operating conditions were changed. The pressure was
established according to the percentage of required relative humidity and vapor pressure of
27
The experiments concluded when the equilibrium was achieved.
Figure 20. Suspension balance experimental installation
28
Table 2. Conditions of the experiments in suspension balance
API weight fraction (%) Polymer Temperature (°C) RH (%)
90 PVAC 40 75
80 PVAC 40 75
70 PVAC 40 75
90 PVAC 30 75
80 PVAC 35 75
90 PVAC 35 75
70 PVAC 35 75
80 PVAC 40 90
80 PVAC 40 60
80 PVAC 40 75
80 PVAC350 40 75
80 PVAC350 40 75
90 PVAC350 40 75
80 Resomer 203s 40 75
70 Resomer 203s 40 75
90 Resomer 203s 40 75
85 PVAC 40 75
75 PVAC 40 75
80 PVAC (Melting) 40 75
70 PVAC (Melting) 40 75
80 PVAC 40 75
80 PVAC 30 75
80 PVAC350 40 75
90 PVAC 40 75
3.2.3 Verification of crystallinity via PXRD and DSC
The PXDR measurements have been carried out by Miniflex 600 from Rigaku (Tokyo, Japan)
in order to determine if the solid dispersions were amorphous right after the spray drying and
29
The quantity of the sample to be analyzed was around 5 mg and was kept on a silica area.
Figure 21. Rigaku Miniflex 600 equipment
The DSC measurements have been performed by DSC Q2000 device manufactured by TA
Instruments (New Castle, DE, USA) in order to determine thermal characteristics such as
degree of crystallinity and melting temperature and enthalpy. These were studied by using TA
Universal Analysis software.
For these measurements, an amount of 4-10 mg was weighted and placed in a standard pan.
Some of the adjustments, such as heating rate, were taken from the equipment manual. The
30
Figure 22. TA Instruments DSC Q-2000 equipment
3.2.5 UV-Vis measurements
In order to verify the final nifedipine concentration of every sample, absorbance was
evaluated. The precise concentration in solution was measured by using a BioSpectrometer®
from Eppendorf (Hamburg, Germany).
The spectrophotometer was set at a specific wavelength. The absorbance of the nifedipine was
measured at 276 nm. The solvent used was acetonitrile. The samples were measured three
31
32
4. Results and discussion
In this section, all the results of the experiments performed are presented. Results from
suspension balance, DSC, PXDR and spectrophotometer are shown.
Besides, the influence of every single factor in the solid dispersion is discussed.
4.1 Modeling the phase behavior of API/polymer-formulations
The modeling of the VLEs was determined in this thesis using the PC-SAFT state
equilibrium. In order to model the system was necessary the pure substance parameters and
binary interaction parameters. The pure substance parameters used for the modeling are listed
in Table 3. In addition to the required pure substance parameters, the binary interaction
parameters kij for NIF-water, NIF-PVAC, NIF- Resomer R203s and the polymers with water
were also required. The parameters used for modeling are listed in Table 4.
Table 3. PC-SAFT NIF parameter
NIF
Molecular weight 346.36
Segment number (mol/g) 0.023474382
Segment diameter (A) 3.581025
Dispersion energy (K) 309.4425
Association energy (-) 1221.582
Association volume (K) 0.02
Melting temperature (°C) 172
33
Table 4. Binary interaction parameters
k
ijk
NIF-water 0.011k
NIF-PVAC -0.0182717k
water-PVAC -0.172k
NIF-Resomer 0.009k
water-Resomer 0The pure substance and binary parameters presented above arise from adjusting the
experimental from [25] to the theoretical model. In the Figure 24 below, it can be seen the
adjustment of the data with the parameters presented above.
0,0 0,2 0,4 0,6 0,8 1,0
0,000 0,005 0,010 0,015 0,020 0,025 0,030
RH (%) wH2O
34 4.2 Predicting the influence of humidity on the phase behavior
Amorphous API formulations are prone to absorb water from the surrounding atmosphere.
This water absorption leads to a crystallization of the NIF, which is a fact to avoid in order to
keep the properties and bioavailability achieved with the amorphous state.
For this purpose, it is an important issue to predict the effect of humidity on the system
behavior. Therefore it was studied the water sorption of the samples depending on the relative
humidity and temperature under which the samples were being tested.
Sorption diagrams for 60%, 75% and 90% of relative humidity are shown in the next Figures
23, 24 and 25.
As it can be observed, there is a vast contrast between the humidities. At a 75% RH, the
sample is able to absorb a maximum value of water of 0,024 in the amorphous state, while at
90% RH it is able to assimilate 0.047 of water and at 60% RH a maximum value of 0.015.
The upper line, represented by triangles, corresponds to the water content accepted by the
amorphous formulation and the lower line corresponds to the water content accepted by the
crystalline formulation. In this way, it is understood the shape of the Figure 10 in which a
maximum water value is obtained in the amorphous state and as the crystallization advances
the water content decreases.
0,0 0,2 0,4 0,6 0,8 1,0
0,00 0,01 0,02 0,03 0,04 0,05 0,06
w
NIF 80%, 40°C, 60% RH
w
NIF
wH2O
35
0,0 0,2 0,4 0,6 0,8 1,0
0,00 0,01 0,02 0,03 0,04 0,05 0,06
wNIF 80%, 40°C, 75% RH
wNIF wH2O
Figure 26. Sorption diagram for sample wNIF 80%, 40°C, 75°RH
0,0 0,2 0,4 0,6 0,8 1,0
0,00 0,01 0,02 0,03 0,04 0,05 0,06
wNIF 80%, 40°C, 90% RH
wNIF wH2O
Figure 27. Sorption diagram for sample wNIF 80%, 40°C, 90°RH
Based on these data, it is easily predictable that there will be a sizeable difference both in time required to reach the equilibrium and initial and final water value between the samples.
36
0,0 0,2 0,4 0,6 0,8 1,0
0,00 0,01 0,02 0,03 0,04 0,05
0,06 wNIF 80%, 35°C, 75% RH
wNIF wH2O
Figure 28. Sorption diagram for sample wNIF 80%, 35°C, 75°RH
0,0 0,2 0,4 0,6 0,8 1,0
0,00 0,01 0,02 0,03 0,04 0,05 0,06
wNIF 80%, 30°C, 75% RH
wNIF wH
2O
Figure 29. Sorption diagram for sample wNIF 80%, 30°C, 75°RH
4.3 Results of suspension balance experiments
The solid dispersions were placed under the desired conditions of temperature and pressure in
the suspension balance.
Firstly, it was decided to set a sample and conditions as a point of reference. This was an NIF
weight fraction of 80%, a temperature of 40 °C and a RH of 75%. And in order to guarantee
its reproducibility, the experiment was repeated twice. In order to have the same
37
The data collected from the balance system are weight of the sample and the cell where the
powder is; it was also weighted the cell empty. From this weight of the cell and powder and
the weight of the cell itself, it can be calculated the water content in the sample.
As it can be seen in Figure 30, both experiments overlap each other reaching the same water
content at the beginning and at the end and achieving the equilibrium at the same time.
0 15 30 45 60 75
0,000 0,005 0,010 0,015 0,020 0,025 0,030 0,035 Water content
(%)
Time (h)
wNIF=80%
wNIF=80%
Figure 30. Overlap of the two independent water sorption measurements obtained for wNIF=0.80 at 40
°C, 75% RH to check the reproducibility of the experiments
Once this was assured, it was proceeded to study the effect of the influencing factors, always
taking these values as reference.
4.3.1 Influence of wNIF
Different weight fractions of the compounds were tested in experiments; these were 90%,
85%, 80%, 75% and 70% of NIF. The temperature and relative humidity were 40°C and 75%,
respectively. The results obtained can be seen in Figure 29.
As it can be observed, there is an evolution in every case. The higher concentration of API in
38
And also the lower concentration of API the sample contains, the longer it takes to
crystallizes.
0 25 50 75 100 125
0,000 0,005 0,010 0,015 0,020 0,025 0,030 0,035 Water content
(%)
Time (h)
w
NIF=0,7 wNIF=0,75
w
NIF=0,80
wNIF=0,85 wNIF=0,9
Figure 31. Evolution of water content along the weight fraction
In order to avoid the crystallization of NIF, it will be always to work with a low API
concentration. However, it could be the case that with that weight fraction does not reach the
recommended dose, in that case it would have to reach a compromise between the weight
fraction and the improved properties achieved and the recommended dose. On the other side,
future investigations would go in the direction of studying lower API weight fractions.
This can be explained, as it was described in 2.3 Influence of RH on the phase behavior, due
to the shape of the curve in Figure 10.
4.3.2 Influence of RH
The effect of the relative humidity in the crystallization was also studied. RH of 90%, 75%
and 60% were analyzed. The conditions under which the experiments were performed were:
wNIF = 0.80, 40°C of temperature and the corresponding RH.
39
15 30 45 60 75 90 105 120
0,000 0,005 0,010 0,015 0,020 0,025 0,030 0,035
60% RH 75% RH
90% RH
Water content (%)
Time (h)
Figure 32. Evolution of water content along RH
As it can be observed in the previous plot, there is a sizable difference between the
experiments both in initial and ending water content and time to reach the equilibrium. The
higher RH, the higher water absorption, which translates into greater molecular mobility,
which leads to greater crystallization [35].
This behavior was also found in [26].
4.3.3 Influence of Temperature
Another influencing factor that has been analyzed is the temperature. The temperatures
studied were 30, 35 and 40 °C with NIF weight fractions of 90, 80 and 70%. The RH was
40
0 25 50 75 100 125 150
0,000 0,005 0,010 0,015 0,020 0,025 0,030 0,035
wNIF=90%,35°C
wNIF=90%,40°C
wNIF=90%, 30°C
Water content (%)
Time (h)
Figure 33. Evolution of water content along temperature for wNIF 90% and 75%RH
As it is observed in Figure 33, the temperature affects the crystallization time. Comparing the
temperatures with our reference of 40°C, it is perceived an appreciable difference between
them.
0 25 50 75 100 125 150
0,000 0,005 0,010 0,015 0,020 0,025 0,030 0,035
wNIF=80%, 35°C
wNIF=80%, 40°C Water content
(%)
Time (h)
wNIF=80%, 30°C
41
In the case of wNIF of 80% shown in Figure 34, the difference is even greater than before as it
was reached a week time without the 30 °C curve began to decline.
For the 70% NIF sample observed in Figure 35, it can be seen a considerable difference again.
In this NIF weight fraction, the experiment was not performed at 30 °C as it would have been
obtained a straight line similar to the 35°C line.
0 25 50 75 100 125 150
0,000 0,005 0,010 0,015 0,020 0,025 0,030 0,035
wNIF=70%, 40°C
wNIF=70%, 35°C
Water content (%)
Time (h)
Figure 35. Evolution of water content along temperature for wNIF 70% and 75%RH
According to the results, the temperature conditions in order to prevent crystal formation are
the lowest possible temperature.
4.3.4 Influence of polymer type
Another influencing factor studied was the polymer type. It was studied 2 polymers:
Resomer® R203 S and PVAC. Regarding to the final polymer, this was investigated 2
different molecular weights. The results can be seen in Figure 36.
As it is shown, there is a contrast between the two polymers in both the crystallization time
and water content. The polymer Resomer® R203 S took less time to crystallize and reached
42
It can be also noticed the different behavior between the polymer with differing molecular
weight although they accomplished the same initial and ending water content.
0 25 50 75
0,000 0,005 0,010 0,015 0,020 0,025 0,030 0,035
Resomer 203s PVAC350
PVAC90
Water content (%)
Time (h)
Figure 36. Evolution of water content along polymer for wNIF 80%, 40°C and 75%RH
4.3.5 Influence of the method to prepare the formulation
The last influencing factor studied was the method used in order to prepare the formulation.
Two forms of preparation were taken into account: spray drying, as the method used in all the
experiments performed, and melting cooling as the method used in real manufacturing.
43
0 25 50 75
0,000 0,005 0,010 0,015 0,020 0,025 0,030 0,035
melt cooling
Water content (%)
Time (h)
spray drying
Figure 37. Evolution of water content along the method to prepare the formulation for wNIF 80%,
40°C and 75%RH
In Figure 37, it can be seen that almost the same initial and ending values of water content are
achieved.
On the other side, the difference in time to crystallize is appreciable. The time needed for the
sample made by melting process is lower than the spray drying sample.
This is an interesting issue since it is question of avoiding crystallization in time, and this
melting cooling is the method used in industry.
Calculation of crystallinity via mass balance
Once the suspension balance experiments were done, from the water content data obtained
from them, it was proceeded to calculate the degree of crystallinity by mass balances.
44
Table 5. Degree of crystallinity of the samples by mass balances
Polymer wNIF (%) T (°C) RH (%) wCrystals (%)
PVAC 90 40 75 89
PVAC 80 40 75 73
PVAC 70 40 75 63
PVAC 90 35 75 86
PVAC 80 35 75 72
PVAC 70 35 75 18
PVAC 90 30 75 90
PVAC 80 30 75 6
PVAC 80 40 90 79
PVAC 80 40 60 74
PVAC350 80 40 75 73
Resomer 203s 90 40 75 87
Resomer 203s 80 40 75 82
Resomer 203s 70 40 75 70
PVAC 85 40 75 76
PVAC 75 40 75 69
PVAC Meltcool 80 40 75 74
PVAC Meltcool 70 40 75 60
As it can be observed in the previous Table 5 the higher degree of crystallinity was reached
always with a wNIF of 90%. It is also noted that the way of preparation of the formulation
(spray drying or melting-cooling) does not interfere in the crystallinity achieved even though
it does interfere in the process of absorbing water.
The progress of the different concentrations in the system can be observed in the next Figure
38 and 39 for the reference sample (wNIF 0.80, 40°C, 75% RH). It is appreciable that the drop
of the API in the liquid phase coincides with the increase of crystal growth and at the same
45
0 10 20 30 40 50 60 70
0,0 0,1 0,2 0,3 0,4 0,5 0,6 0,7 0,8 0,9 1,0 W Time (h)
wPol in L
wAPIF in L
wH2O L
Figure 38. Concentration of compounds in the liquid phase for reference sample (wNIF 80%, 40°C,
75%RH)
0 10 20 30 40 50 60 70
0,00 0,01 0,02 0,03
wWATER wAPIL
Time (h) wCRYST wCRYST wAPIL wWATER 0,0 0,2 0,4 0,6 0,8 1,0 0,0 0,2 0,4 0,6 0,8 1,0
Figure 39. Evolution of concentration of crystals, water and API for reference sample (wNIF 80%,
40°C, 75%RH)
According to what was seen in previous sections, as the water content decreases, the degree of
crystallization of the sample increases so the NIF content in the liquid phase decreases, as it
can be observed in Figure 39.
The plots of the development of concentrations for the rest of the samples can be seen in
46 4.4 Results of PXRD and DSC-measurements
During this chapter it will be shown the results of the PXRD and DSC measurements. By
performing these experiments, it was wanted to guarantee the amorphous state of the sample
right after the spray drying and before the experiment in the suspension balance and the
crystalline state after the suspension balance. It was also wanted to calculate the melting
enthalpy and therefore verify the degree of crystallinity.
In the next Figures 40 and 41, it is shown the results from PXRD in which the amorphous and
crystalline states of the reference sample can be seen, that is wNIF of 80%, 40°C and 75% of
RH.
Figure 40. PXRD pattern of amorphous state of sample wNIF 80%
![Figure 1. Scheme of spray drying method [6]](https://thumb-us.123doks.com/thumbv2/123dok_es/5475408.725924/20.892.175.711.625.938/figure-scheme-spray-drying-method.webp)
![Figure 2 Hot melt extrusion process [7]](https://thumb-us.123doks.com/thumbv2/123dok_es/5475408.725924/22.892.174.720.205.428/figure-hot-melt-extrusion-process.webp)
![Figure 6. Behavior of solubility against temperature and API weight fraction [10]](https://thumb-us.123doks.com/thumbv2/123dok_es/5475408.725924/25.892.234.660.256.567/figure-behavior-solubility-temperature-api-weight-fraction.webp)
![Figure 9. Behavior of NIF solubility [10]](https://thumb-us.123doks.com/thumbv2/123dok_es/5475408.725924/27.892.46.841.142.349/figure-behavior-of-nif-solubility.webp)
![Figure 10. Behavior of water content and phases during crystallization for a w NIF 70% [10]](https://thumb-us.123doks.com/thumbv2/123dok_es/5475408.725924/28.892.131.770.485.730/figure-behavior-water-content-phases-crystallization-w-nif.webp)
![Figure 13. Characteristic sigmoidal curve followed by crystallization [16]](https://thumb-us.123doks.com/thumbv2/123dok_es/5475408.725924/35.892.169.725.244.526/figure-characteristic-sigmoidal-curve-followed-crystallization.webp)
![Figure 16. Molecular structure of NIF polymorphs [23]](https://thumb-us.123doks.com/thumbv2/123dok_es/5475408.725924/38.892.227.684.435.637/figure-molecular-structure-of-nif-polymorphs.webp)

