A novel modulatory mechanism of transforming growth factor ss signaling through decorin and LRP 1
Texto completo
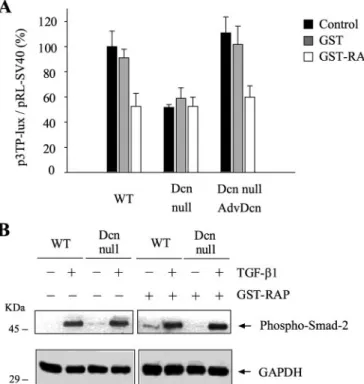
(2) LRP-1-decorin Are Required for TGF- Signaling activation of the Smad pathway and is affected by PI3K inhibitors. This work unveils a new regulatory role for decorin and LRP-1 in TGF- signaling.. JUNE 29, 2007 • VOLUME 282 • NUMBER 26. JOURNAL OF BIOLOGICAL CHEMISTRY. 18843. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. EXPERIMENTAL PROCEDURES Cell Culture—The mouse skeletal muscle cell line C2C12 (ATCC) (28) was grown and induced to differentiate as described (29). Decorin null (Dcn null) myoblasts have been previously described and characterized (18). Adenoviral Infection—Myoblasts were plated at a density of 10,000 cells/cm2 in 6-well plates. After 4 h, myoblasts were infected with 500 plaque-forming units/cell of an adenovirus containing the human sequence of decorin (Adv-Dcn) (18) in Dulbecco’s modified Eagle’s minimal essential medium containing 2% heat-inactivated fetal bovine serum (FBS). After 90 min of incubation, standard medium (Dulbecco’s modified Eagle’s minimal essential medium, 10% FBS) was added, and incubation was continued for an additional 24 h. Transfection assays were performed in fresh growth medium. Transient Plasmid Transfection— FIGURE 1. Dcn null myoblasts are less responsiveness to TGF-, whereas re-expression of decorin recovers its sensitivity to TGF-. Wild type (WT), Dcn null, and Dcn null myoblasts infected with Adv-Dcn The following TGF- responsive were transiently transfected with plasmids containing sequence for p3TP-lux (A), pCTGF-luc (B), and constructs were used: p3TP-Lux p4X-SBE-luc (C), in all the cases together with a plasmid containing pRL-SV40 sequence. TGF-1 (1.0 ng/ml for p3TP-lux and p4XSBE-luc, or 5.0 ng/ml for pCTGF-luc) was added, and after 24 h, the cells were (30), pCTGF-Luc (kindly donated processed, and luciferase activities were determined. Wild type values correspond to 100%. The values by Dr. A. Leask, Royal Free and Unicorrespond to the mean ⫾ standard deviation of three independent experiments. D, the cells were incu- versity College Medical School, bated with the indicated amount of TGF-1 and the amount of integrin 1 determined by Western blot. The numbers correspond to the integrin 1/tubulin determined by densitometric analysis. Note that in London, UK), p4X-SBE-luc (31), Dcn null infected with Adv-Dcn and incubated with 5 ng/ml TGF-1, the loading was less as evidenced by and pMyo-Luc (18). pAct-EF Smadthe amount of tubulin. E, total RNA was isolated from wild type and Dcn null myoblasts previously incu7-Myc (kindly donated by Dr. K. bated with or without TGF-1 (1.5 ng/ml) for 24 h and blotted with a specific probe for biglycan (61). The Okazaki, Department of Molecular ratio of byglican/tubulin is shown at the bottom of the figure. Biology, Biomolecular Engineering Research Institute, Furuedai, Suita cells (21, 22). We have recently demonstrated that decorin Osaka, Japan) and pDN-TGF--RII-HA (kindly donated by Dr. binds and that it is endocyted by the low density lipoprotein J. Massagué, Sloan Kettering, Howard Hughes Medical Instireceptor-related protein (LRP-1) (23). LRP-1 is a giant receptor tute, New York, NY) were used to overexpress Smad-7-Myc that binds, internalizes, and mediates the degradation of several and a negative dominant mutant of TGF- receptor II (DNligands (24). The folding process of the receptor in the endo- TGF--RII-HA) respectively. Briefly, cells were plated in plasmic reticulum requires the participation of a 39-kDa recep- 24-well plates until they reached 60% confluence. Cells were tor chaperone, the receptor-associated protein (RAP) (25). This then incubated in Opti-MEM I containing 1 g of each plasmid, protein competes efficiently with the binding of decorin to 0.02 g of pRL-SV40, 2 l of PLUS reagent, and 1 l of LipoLRP-1 at the cell surface (23). To study the possible role of fectamine. After 6 h, FBS was added to the medium, and the LRP-1 in the modulation of TGF- signaling by decorin, we cells were cultured for a further 12 h. Medium was changed to decided to study its role in myoblasts since it has been charac- fresh growth medium, and the following reagents were added: TGF-1 dissolved in 0.5% FBS; GST or GST-RAP in phosphateterized with regard to TGF- response (18, 26, 27). Here we show that decorin and LRP-1 modulate TGF- buffered saline (23), or inhibitors of p38MAPK activity responses. This novel function for LRP-1 and decorin requires (SB203580, from Pierce), TGF- receptor I kinase activity.
(3) LRP-1-decorin Are Required for TGF- Signaling. 18844 JOURNAL OF BIOLOGICAL CHEMISTRY. VOLUME 282 • NUMBER 26 • JUNE 29, 2007. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. (1:5000) (Sigma-Aldrich); or mouse anti-glyceraldehyde-3-phosphate dehydrogenase (1:2000) (Chemicon, Temecula, CA); or with rabbit anti-HA (1/2000), rabbit anti-TGF-RI (1:500), rabbit anti-TGF--RII (1:500), rabbit anti-myogenin (1:100), rabbit anti-integrin 1 (1:1000), goat anti-Smad-2 (1:500); mouse anti-Smad-4 (1:1000) or mouse anti-Myc (1:1000), all from Santa Cruz Biotechnology, Santa Cruz, CA. All immunoreactions were visualized by enhanced chemiluminescence (Pierce). RNA Isolation and Northern Blot Analysis—Total RNA (10 g/lane) was isolated from cell cultures at the indicated days of differentiation using TRIzol. RNA samples were electrophoresed in 1.2% agarose/ formaldehyde gels, transferred to Nytran membranes, and hybridized with probes for mouse Smad-7, biglycan, or tubulin during 3 h to 65 °C, and the probe for mouse connective tissue growth factor (CTGF) was incubated during 20 h at 42 °C. The probe for Smad-7 was obtained by digestion of pActEF-Smad7-Myc plasmid, which has the full mouse FIGURE 2. Smad-2 phosphorylation and Smad-4 translocation to the nucleus is not affected in Dcn null cDNA of Smad-7, with NheI and myoblasts. A, Dcn null myoblasts were incubated with the indicated amounts of TGF-1 for 30 min. Cells were lysed and immunoblotted for phospho-Smad-2 and GADPH. Standard molecular masses are indicated. WT, EcoRV. The probe for tubulin has wild type. B, wild type and Dcn null myoblasts were incubated with the indicated amount of TGF-1 for 60 min been described previously (18). and processed for indirect immunofluorescence for Smad-4. The bar corresponds to 10 m. C, wild type and The probe for CTGF was obtained Dcn null myoblast were or were not infected with Adv-Dcn and treated or untreated with TGF-1 (1.0 ng/ml) for 30 min. Cells were lysed and immunoprobed for phospho-Smad-2 or GADPH. Molecular mass standards are by PCR reactions using specific shown on the left. primers. Immunofluorescence Microscopy— (SB431542, from Sigma-Aldrich), MEK1/2 activity (U0126, LRP expression and distribution and Smad-4 intracellular from Promega, Madison, WI), insulin growth factor receptor I localization in C2C12 myoblasts was analyzed by indirect (IGFR-I) kinase activity (AG1024, from Calbiochem), or PI3K immunofluorescence. Cells were grown on glass coverslips and activity (wortmannin and LY294002, from Sigma-Aldrich). then fixed in 3% paraformaldehyde, permeabilized with 0.05% Dual Luciferase activity assays (Promega) were performed after Triton X-100, and incubated for 1 h with 1:100 mouse antiLRP-1 antibody, directed against the cytoplasmic tail of human 6 or 24 h depending on the experiment. Immunoblot Analysis—For immunoblot analyses, cell LRP-1 (Calbiochem) or 1:100 mouse anti-Smad-4 (Santa Cruz extracts obtained from myoblasts were prepared in 50 mM Tris- Biotechnology). The incubation buffer was 50 mM Tris-HCl, pH HCl, pH 7.4, 0.1 M NaCl, 0.5% Triton X-100 with a mixture of 7.7, 0.1 M NaCl, and 2% bovine serum albumin. After buffer protease inhibitors and 1 mM phenylmethylsulfonyl fluoride. removal and several washes with the above buffer, bound antiFor analysis of phosphorylated proteins, cell extracts were pre- bodies were detected by incubating the cells for 30 min with pared in radioimmune precipitation buffer (RIPA) as described 1:100 affinity-purified fluorescein isothiocyanate-conjugated previously (32). Aliquots were subjected to SDS gel electro- anti-mouse antibodies (Pierce Biotechnology). For nuclear phoresis in 5 or 10% polyacrylamide gels, electrophoretically staining, sections were incubated with 1 g/ml Hoechst 33258 transferred onto nitrocellulose membranes (Schleicher & in phosphate-buffered saline for 10 min. After rinsing, the secSchuell), and probed with rabbit anti-LRP extracellular domain tions were mounted with fluorescent mounting medium (Dako (1:1000) as described previously (33); rabbit anti-phospho- Corp.) under glass coverslips, viewed, and photographed with a Smad-2 (1:1000) (Calbiochem), rabbit anti-phophoSmad-3 Nikon Eclipse microscope equipped for epifluorescence. (1:1000) (BIOSOURCE); rabbit anti-Smad-3 (1:250) (Zymed Short Interfering RNA (siRNA) Transfection—Annealed Laboratories Inc., San Francisco, CA); mouse anti-␣-tubulin siRNA specific for LRP-1 and control siRNA were previously.
(4) LRP-1-decorin Are Required for TGF- Signaling. RESULTS Decorin Is Required for TGF- Response without Changes in the Smad-dependent Pathway—We have previously shown that Dcn null myoblasts present a decreased FIGURE 3. Decorin-dependent TGF- signaling requires phosphorylation of Smad-2 or -3 through the response to TGF-1 when comTGF- transducing receptors. Wild type (WT) and Dcn null myoblasts were transfected with plasmids containing sequence for p3TP-lux together with pRL-SV40. A, wild type and Dcn-null myoblasts were incubated pared with wild type myoblasts, with the specific inhibitor for TGF--dependent serine/threonine kinase activity of TGF--RI, SB431542 (5 M), (18). Now, we have investigated this or vehicle alone Me2SO (DMSO). In the right panel, the inhibitory effect on Smad-2 and -3 phosphorylation by SB431542 is shown by immunoblot for the corresponding Smad proteins and GAPDH in wild type and Dcn null phenomenon, evaluating three myoblasts. B, the cells were transfected with increasing concentrations of a plasmid containing the sequence TGF--inducible reporters: p3TPfor Smad-7 containing a Myc epitope. In the right, the corresponding immunoblots for Myc epitope and tubulin lux, which contains the promoter are shown. C, myoblasts were transfected with increasing concentrations of a plasmid containing the sequence for a dominant negative form of TGF--RII containing a HA epitope (DN-TGF--RII-HA). Immunoblots for HA region of plasminogen activator epitope and tubulin are shown (right). In A–C, the activities in response to TGF-1 were determined after 24 h. inhibitor (34); pCTGF, a reporter Values correspond to mean ⫾ standard deviation of three independent experiments. Non-transfected wild type and Dcn null myoblasts correspond to 100%. Right, top to bottom, the inhibitory effect on Smad-2 and -3 containing the promoter region of phosphorylation by SB431542 is shown by immunoblot for the corresponding Smad forms and GAPDH. The CTGF (35); and p4X-SBE-luc, a absence of recovery of luciferase activity by re-expression of decorin is shown in myoblasts overexpressing reporter containing four specific Smad-7 or DN-TGF--RII-HA. The corresponding immunoblots for Myc epitopes, HA, and tubulin are shown, binding sites for Smad proteins (31). and the migration positions of molecular mass standards are shown on the left. The three reporter activities were reduced in response to TGF- in described (23). Briefly, for transfection, myoblasts were seeded Dcn null when compared with wild type myoblasts (Fig. 1, into six-well plates until they reached 70% confluence. Subse- A–C). The same effect was observed for the amount of integrin quently, cells were incubated during 6 h in 800 l of Opti-MEM 1, a TGF-1-inducible protein (Fig. 1D). The decreased I containing siRNA for LRP-1 or control siRNA plus 8 l of responses to TGF-1 were specific to the absence of decorin JUNE 29, 2007 • VOLUME 282 • NUMBER 26. JOURNAL OF BIOLOGICAL CHEMISTRY. 18845. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. Lipofectamine 2000 (Invitrogen). For experiments with p3TP-Lux or pMyo-Luc plasmid reporters, these were co-transfected with siRNA. Following transfection, FBS was added to the medium, and the cells were cultured for a further 24 h. Then, cells were analyzed through immunofluorescence assays of Smad-4 or LRP-1 or harvested for immunoblot analysis of LRP-1, myogenin, or integrin 1. Labeling of Cultures—Cells were infected with Adv-Dcn and incubated for 18 h in sulfate- and serumfree Dulbecco’s modified Eagle’s minimal essential medium/F12 with 100 Ci/ml H2[35S]O4 (25 mCi/ml, PerkinElmer Life Sciences) (18). These conditioned media were concentrated on a DEAE-Sephacel column pre-equilibrated in 10 mM Tris-HCl, pH 7.4, 0.2 M NaCl, and 0.1% Triton X-100, and samples were eluted with 1 M NaCl and analyzed by 4 –12% SDS-PAGE. Gels were dried and exposed to phosphorimaging system. Protein Determination—Proteins were determined in aliquots of cell extracts using the bicinchoninic acid protein assay kit (Pierce) using bovine serum albumin as standard..
(5) LRP-1-decorin Are Required for TGF- Signaling. 18846 JOURNAL OF BIOLOGICAL CHEMISTRY. FIGURE 4. RAP inhibits TGF--dependent activity without affecting phosphorylation of Smad-2. A, wild type (WT), Dcn null, and Dcn null myoblasts infected with Adv-Dcn were transiently transfected with plasmids containing p3TP-lux and pRL-SV40 sequences and incubated with or without GST-RAP or GST (1 M); after 6 h of TGF-1 (1.0 ng/ml) treatment, cells were lysed, and reporter activities determined. Values correspond to mean ⫾ standard deviation of three independent experiments. B, in the same cells as in A, the amount of Smad-2 phosphorylated and GADPH was determined by immunoblot. Molecular mass standards are indicated on the left.. decorin exerts in response to TGF-, we determined TGF-dependent p3TP-Lux activity in the presence or absence of RAP, a protein that inhibits the binding and endocytosis of decorin by LRP-1 (23). RAP inhibited about 50% of TGF-1-dependent p3TP-Lux activity in wild type myoblasts (Fig. 4A) similar to the decrease of TGF- activity in Dcn null cells. Importantly, the inhibitory effect of RAP depends on decorin because in Dcn null myoblasts, RAP has no effect (Fig. 4A). The inhibitory effect by RAP was reversible (supplemental Fig. 3A). This effect was dependent on the presence of decorin because in Dcn null myoblasts that re-express this proteoglycan, RAP inhibited the induction of p3TP-Lux in response to TGF-1 (Fig. 4A). RAP did not have any effect on Smad-2 phosphorylation in response to TGF-1 in wild type and Dcn null myoblasts (Fig. 4B), supporting the idea that Smad-dependent TGF- signaling is not affected in Dcn null myoblasts. Levels of LRP-1 in wild type and Dcn null myoblasts were equivalent (supplemental Fig. 3B). To prove the participation of LRP-1 in the regulatory mechanism that decorin exerts in the TGF- response in myoblasts, we used siRNA for LRP-1. A strong decrease of LRP-1 protein was observed when compared with siRNA control transfected cells (Fig. 5A). The strong decrease of LRP-1 was confirmed by indirect immunofluorescence (supplemental Fig. 3C). To determine whether the absence of LRP-1 has an effect in the response to TGF-, we evaluated: p3TP-Lux activity; the amount of integrin 1; and the amount of CTGF messenger transcripts in wild type and Dcn null myoblasts transfected with VOLUME 282 • NUMBER 26 • JUNE 29, 2007. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. since its re-expression, using an adenovirus containing AdvDcn, restored the TGF--dependent activity to wild type levels (Fig. 1, A–D). The diminished response was observed at different TGF-1 concentrations (supplemental Fig. 1, A and B). Reexpression of decorin, after the infection with the Adv-Dcn in Dcn null myoblasts, shows this proteoglycan as the main radiolabeled biosynthetic product (supplemental Fig. 1C). These results suggest that decorin is required for part of the response to TGF- in myoblasts. The proteoglycan biglycan, highly related to decorin, has been shown to be a target of TGF- (36). Fig. 1E shows the amount of biglycan messenger transcripts in wild type and Dcn null myoblasts in response to TGF- for 24 h. A diminished response to TGF-, when compared with wild type myoblasts, was observed in Dcn myoblasts. The levels of TGF--RI and TGF--RII, the binding of 125ITGF-1 to TGF--RI and TGF--RII, as well as the amount of Smad-2, -3, -4, and -7 were similar in the Dcn null when compared with wild type myoblasts (supplemental Fig. 2, A and B). The phosphorylation of Smad-2 induced by different amounts of TGF-1, as well as the kinetic, are normal when compared with wild type cells (Fig. 2A, and see supplemental Fig. 2C). Indirect immunofluorescence analyses showed that the nuclear accumulation of Smad-4 in response to different TGF-1 concentrations was also similar in both cell types (Fig. 2B). Importantly, the re-expression of decorin in Dcn null myoblasts by infection with Adv-Dcn did not affect the amount of phosphoSmad-2 (Fig. 2C), an experimental situation that totally recovers TGF- responses in Dcn null myoblasts (Fig. 1, A–D). These results clearly indicate that although Dcn null myoblasts are less responsive to TGF-, the Smad pathway is unaffected. Decorin Requires an Active Smad Signaling Pathway to Allow Maximal TGF- Response—To examine the contribution of the Smad pathway with regard to the role of decorin in the TGF- response, we used a specific inhibitor of TGF--RI kinase activity (SB431542) (Fig. 3A), overexpression of Smad-7, an inhibitor of TGF- phosphorylation of Smad-2 and -3 (Fig. 3B), or a dominant negative mutant of TGF- receptor II (DNTGF--RII) (Fig. 3C) in wild type and Dcn null myoblasts. These three approaches, independently, produced a strong decrease in the TGF-1-dependent p3TP-Lux activity in wild type and Dcn null myoblasts, which was dependent on the expression levels of Smad-7 or DN-TGF--RII. Under these treatments, re-expression of decorin in Dcn-null myoblasts did not recover TGF--dependent activity (Fig. 3A, left panel, and B and C, right panels). The effect of the SB431542 inhibitor on Smad-2, -3 phosphorylation and the overexpression of Smad-7 and DN-TGF--RII are shown (Fig. 3A, right panel, and B and C, left panels). The above results suggest that decorin requires an active Smad pathway to allow maximal TGF- response. Decorin Requires LRP-1 to Exert Its Effect on TGF- Signaling—Decorin is a soluble proteoglycan internalized by different cell types, including endothelial and myogenic cells. One possibility to explain how decorin positively regulates the TGF- response in myoblasts is through its interaction with a specific cell surface receptor. A candidate for this function is LRP-1, the endocytic receptor for decorin, as we have recently shown (23). To evaluate whether LRP-1 participates in the regulation that.
(6) LRP-1-decorin Are Required for TGF- Signaling. ingly, in Dcn null myoblasts, the absence of LRP-1 did not affect any of the parameters evaluated (Fig. 5B and C, and supplemenJUNE 29, 2007 • VOLUME 282 • NUMBER 26. In this study, we have shown that TGF- signaling depends on decorin and LRP-1. The proteoglycan decorin is a pleiotroJOURNAL OF BIOLOGICAL CHEMISTRY. 18847. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. tal Fig. 4). When Dcn null myoblasts re-express decorin, the absence of LRP-1 inhibited p3TP-Lux activity and the amounts of integrin 1 and CTGF messenger transcripts (Fig. 5, B and C, and supplemental Fig. 4). Therefore, decorin did not re-establish maximum levels of TGF- response in the absence of LRP-1. Finally, the absence of LRP-1 did not have any effect on the nuclear accumulation of Smad-4 in response to TGF-1 in wild type or Dcn null myoblasts (Fig. 5D). These results suggest that decorin could exert its effect on TGF- signaling through LRP-1. Inhibitors of PI3K Pathway Affect the Decorin-LRP-1 Component of the TGF- Response—The above results indicate that part of the response to TGF- is modulated by the presence of decorin and LRP-1. To study which pathway(s) might be implicated in this decorin-LRP-1 modulatory effect, we used specific inhibitors of PI3K, p38MAPK, MEK1/2, and IGFR-I kinase activities on the response to TGF- of wild type and Dcn null myoblasts. Only the inhibitor of the PI3K pathway LY294002 affected the decorinLRP-1 component of the response to TGF- determined by the p3TPlux reporter activity (Fig. 6A). Inhibitors of p38MAPK (Fig. 6B) did not affect the p3TP-lux activity, and inhibitors of MEK1/2 or IGFR-I kinase activity did affect the TGF-dependent reporter activity in both cell types independent of decorin expression (Fig. 6, C and D). Fig. 7A shows that re-expression of decorin FIGURE 5. LRP-1 is required for TGF--dependent signaling. A, wild type myoblasts were transfected with siRNA control or for LRP-1. The inhibition of LRP-1 expression is shown by immunoblot for LRP-1 and tubulin. using Adv-Dcn did not revert the response. Finally, the B, wild type (WT) myoblasts were transfected with siRNA control or for LRP-1 together effect of two PI3K inhibitors (wortwith the TGF- responding reporter p3TP-lux and pRL-SV40 as control of transfection efficiency, and after 24 h, the activity in response to TGF-1 was determined. Values for wild type cells treated with Lipofectamine mannin and LY294002) on the (control) and TGF-1 (1.0 ng/ml) correspond to 100%. C, wild type, Dcn null and Dcn null myoblasts infected TGF- response. Finally, the absence with Adv-Dcn were transfected with siRNA control or for LRP-1 and incubated with or without TGF-1 (1.0 of LRP-1 did not revert the effect of ng/ml). The amount of integrin 1 and tubulin was determined by immunoblot analyses. At the bottom of the figure, the integrin 1/tubulin ratio are shown. D, wild type and Dcn null myoblasts were transfected with LY294002 on the TGF-1 response in siRNA control or LRP-1 and incubated with or without TGF-1 (1.0 ng/ml) for 60 min. Smad-4 localization was wild type or Dcn null myoblasts (Fig. determined by indirect immunofluorescence. The position of standard molecular mass (in kDa) is indicated on the left in A and C. In D, the bar corresponds to 10 m. In B and D, values correspond to mean ⫾ standard 7B). These results suggest that the PI3K pathway may be involved in the deviation of three independent experiments. modulatory response to TGF- a siRNA for LRP-1 or siRNA control. The decrease of LRP-1 mediated by the interaction between LRP-1 and decorin. protein levels diminished these three parameters in wild type myoblasts (Fig. 5, B and C, and supplemental Fig. 4A). Interest- DISCUSSION.
(7) LRP-1-decorin Are Required for TGF- Signaling. pic molecule that has the ability to interact with several ligands, among them TGF- (12). It has been shown that as a result of this interaction, decorin can inhibit as well as activate TGF-dependent signaling depending on the cell type (12, 37). It inhibits TGF--dependent signaling in mesangial cells (38), glioma cells (39), and differentiated myotubes (30) and stimulates this signaling in osteoblasts (16) and non-differentiated myoblasts (18). Several proteoglycans have a well established role as co-receptors (19, 40). Because decorin is required for TGF- signaling activity (18) and LRP-1 has the ability to directly bind and subsequently internalize decorin (23), we hypothesized that to participate in TGF- signaling, decorin interacts with LRP-1. In this study, we present experimental evidence that LRP-1, a receptor located on the myoblast surface, is required for this decorin-dependent TGF- signaling. The role of decorin in this respect was evaluated in myoblasts that do not synthesize decorin, determining the transcriptional activity of four different promoters that respond to TGF-; p3TP-Lux, pCTGF-luc, p4xSBE-luc, and pMyo-Luc; and a TGF- target protein, integrin 1. TGF- showed a decreased response in Dcn null myoblasts, but it returned to wild type levels when re-expressing decorin using the Adv-Dcn. In Dcn null myoblasts TGF--R1 and TGF--RII, the amounts of Smad-2, -3,-4, and -7, the kinetics and titration curve of phosphorylation of Smad-2, together with the nuclear translocation of Smad-4, in response to TGF-, were unaltered in Dcn null when compared with wild type myoblasts. Importantly, when Dcn null myoblasts were induced to re-express decorin, the amount of phosphorylated Smad-2 remained unaltered. All these results suggest that the Smad-dependent path-. 18848 JOURNAL OF BIOLOGICAL CHEMISTRY. FIGURE 7. Inhibition of PI3K pathway affect component of TGF- when decorin and LRP-1 are present. A, wild type (WT) and Dcn null myoblasts were transiently transfected with plasmids containing p3TP-lux and pRLSV40 reporters and co-incubated with TGF-1 (1.0 ng/ml) for 24 h in the presence of wortmannin (1 M) or LY294002 (10 M). When corresponding myoblasts were infected with Adv-Dcn. Then all the cells were lysed, and luciferase activity was determined. The values correspond to correspond to mean ⫾ standard deviation of three independent experiments. B, wild type myoblasts were transfected with siRNA control or LRP-1 and co-incubated with TGF-1 (1.0 ng/ml) for 24 h in the presence of LY294002 (10 M). After treatment, cells were lysed, and luciferase activity was determined. Values correspond to mean ⫾ standard deviation of three independent experiments.. way was not affected in Dcn null myoblasts. These results suggest that another component might be regulating decorindependent TGF- signaling. We evaluated the role of LRP-1 using RAP, an inhibitor of decorin binding to LRP-1 and siRNA for LRP-1 (23). RAP and inhibition of LRP-1 expression, using siRNA, inhibited TGF--dependent signaling in wild type myoblasts to the levels observed in Dcn null myoblasts, without effect in the remaining TGF- response observed in this cell type. The inhibition of TGF--dependent transcription by RAP or siRNA for LRP-1 in Dcn null myoblasts that re-express decorin was attained at levels comparable with those observed in uninfected Dcn null myoblasts. In all these cases, no effect on the phosphorylation of Smad-2 or nuclear translocation of Smad-4 was detected, confirming that the Smad pathway was not directly involved in the decreased response to TGF- observed in the absence of decorin. The dependence of TGF- signaling on decorin and LRP-1 seems to be opposite to the described mechanism of decorin-mediated inhibition of TGF- signaling with the subsequent phosphorylation of Smad-2 at a key regulatory site and the sequestration of Smad-4 in the nucleus (41). Although TGF-, a strong inhibitor of myogenesis (26, 27), is present during myogenesis in development or muscle regenerVOLUME 282 • NUMBER 26 • JUNE 29, 2007. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. FIGURE 6. PI3K inhibitors affect the decorin-LRP-1 component of TGF- signaling. Wild type (WT) and Dcn null myoblasts were transiently transfected with plasmids containing p3TP-lux and pRL-SV40 reporters and coincubated with TGF-1 (1.0 ng/ml) for 24 h in the presence of LY294002 (10 M) for PI3K activity (A), SB 203580 (20 M) for p38MAPK activity (B), U0126 (10 M) for MEK1/2 activity (C), and AG1024 (10 M) for IGFRI activity (D). The values correspond to correspond to mean ⫾ standard deviation of three independent experiments. DMSO, Me2SO..
(8) LRP-1-decorin Are Required for TGF- Signaling. JUNE 29, 2007 • VOLUME 282 • NUMBER 26. affect TGF--induced activity in decorin-independent fashion in myoblasts. The binding of growth factors to proteoglycans and the consequent modulation of growth factor activities represent an important conceptual advance in the field. In myoblasts, we have shown that the expression of decorin is up-regulated during skeletal muscle differentiation (58), and as shown in this study, it is a requirement for myoblasts to respond to TGF- through the interaction with LRP-1. On the other hand, we have shown that decorin during skeletal muscle differentiation diminished TGF- binding to its receptors and signaling (30). This apparent discrepancy can be explained as follows; in myoblasts, most of the decorin interacts with cell receptors, such LRP-1 (23). During skeletal muscle differentiation, the amount of LRP-1 on the cell surface diminished substantially (23), the ECM became more structured, and several proteoglycans (59, 60) including decorin are incorporated, sequestering TGF-, and as a consequence, decreasing TGF- binding to cell surface receptors signaling (30). Therefore, the binding of growth factors to proteoglycans and the interaction of these complexes with the plasma membrane or ECM would be critical for its regulatory action. It is provocative to suggest that the requirement of LRP-1 and decorin for TGF- signaling, as shown in this study, is part of a situation, whereas these traditionally believed ECM molecules and endocytic receptors are involved in intricate signaling mechanisms that might be relevant to many cellular responses and be involved in many diseases. Acknowledgments—We thank Dr. Marı́a Paz Marzolo for suggestions and some reagents and Dr. Juan Larraı́n, Valeria Mezzano, and Cecilia Vial for critical reading of the manuscript. The MIFAB was financed in part by Ministerio de Planificación y Cooperación (Chile).. REFERENCES 1. Massague, J. (1998) Annu. Rev. Biochem. 67, 753–791 2. Gray, P. C., Bilezikjian, L. M., and Vale, W. (2001) Mol. Cell. Endocrinol. 180, 47–53 3. Massague, J. (2000) Nat. Rev. Mol. Cell. Biol. 1, 169 –178 4. Lopez-Casillas, F., Riquelme, C., Perez-Kato, Y., Ponce-Castaneda, M. V., Osses, N., Esparza-Lopez, J., Gonzalez-Nunez, G., Cabello-Verrugio, C., Mendoza, V., Troncoso, V., and Brandan, E. (2003) J. Biol. Chem. 278, 382–390 5. Massague, J., Blain, S., and Lo, R. (2000) Cell 103, 295–309 6. Piek, E., Heldin, C., and Ten Dijke, P. (1999) FASEB. J. 13, 2105–2124 7. Derynck, R., and Zhang, Y. E. (2003) Nature 425, 577–584 8. Imamichi, Y., Waidmann, O., Hein, R., Eleftheriou, P., Giehl, K., and Menke, A. (2005) Biol. Chem. 386, 225–236 9. Lien, S. C., Usami, S., Chien, S., and Chiu, J. J. (2006) Cell. Signal. 18, 1270 –1278 10. Yu, L., Hebert, M. C., and Zhang, Y. E. (2002) EMBO J. 21, 3749 –3759 11. Rodriguez-Barbero, A., Dorado, F., Velasco, S., Pandiella, A., Banas, B., and Lopez-Novoa, J. M. (2006) Kidney Int. 70, 901–909 12. Iozzo, R. V. (1999) J. Biol. Chem. 274, 18843–18846 13. Schonherr, E., Sunderkotter, C., Iozzo, R. V., and Schaefer, L. (2005) J. Biol. Chem. 280, 15767–15772 14. Tufvesson, E., and Westergren-Thorsson, G. (2002) FEBS Lett. 530, 124 –128 15. Moscatello, D. K., Santra, M., Mann, D. M., McQuillan, D. J., Wong, A. J., and Iozzo, R. V. (1998) J. Clin. Investig. 15, 406 – 412 16. Takeuchi, Y., Kodama, Y., and Matsumoto, T. (1994) J. Biol. Chem. 269, 32634 –32638. JOURNAL OF BIOLOGICAL CHEMISTRY. 18849. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. ation, these processes occur successfully (42, 43), suggesting that mechanisms to attenuate TGF- signaling might exist. One of these mechanisms could be LRP-1-dependent; we have determined that the amount of LRP-1 decreases substantially during skeletal muscle differentiation (23). Decorin-LRP-1-dependent TGF- signaling does not involve changes in the levels, phosphorylation, or nuclear translocation of several constituents of the Smad-dependent pathway, but our experiments overexpressing the inhibitory Smad-7, the use of a specific inhibitor of the kinase activity of TGF--RI, or using a dominant negative form of the TGF--RII strongly suggest that these components are required for this decorin-LRP-1 dependence. Thus, it is plausible to propose that the component of the response mediated by the TGF-, which depends on decorin and LRP-1, requires the phosphorylation of Smad-2 and -3 and translocation to the nucleus associated with Smad-4. There is growing evidence that indicates that TGF- also signals in a Smad-independent fashion (7). Besides Smad-mediated transcription, TGF- activates other signaling cascades, including MAPK pathways. Some of these pathways regulate Smad activation, but others might induce responses not related to transcription (7). TGF- can activate the ERK (44), p38 MAPK (45), IGFR-I (13), and PI3K (46) kinase pathways. By the use of specific inhibitors of those kinases, we have found that only inhibitors of PI3K affected TGF--dependent signaling in wild type myoblasts without an effect in Dcn null myoblasts, suggesting that the PI3K pathway might be involved in this decorin-LRP-1-dependent TGF- signaling. Furthermore, reexpression of decorin in Dcn null myoblasts, a situation that recovers TGF- signaling, was affected by the use of PI3K inhibitors. There are few examples in which PI3K/Akt enhances expression of TGF- target proteins in response to this growth factor (9, 47, 48). Further studies are required to clarify this point. LRP-1, through its large ectodomain, which contains four ligand-binding domains, binds (among other proteins) multiple ECM molecules, metalloproteinases, and plasminogen activator (24). LRP-1 has generally been recognized as an endocytic receptor involved in clearance and cellular degradation of ligands. However, LRP-1 also regulates signaling cascades by binding growth factors such as platelet-derived growth factor (49), CTGF (50), and TGF- (51, 52). Interestingly, several of these molecules also bind decorin (53–55). Type V TGF- receptor was found to be identical to LRP-1 (56) and mediates its response upon stimulation of insulin-like growth factorbinding protein 3 (IGFBP-3) (56, 57). Many carcinoma cells produce little or no LRP-1 and do not respond to growth inhibition induced by IGFBP-3 or TGF-. Besides TGF- (17), decorin binds to the insulin-like growth factor-I (13) and interacts with tumor necrosis factor-␣ (14). Decorin is also known to cause rapid phosphorylation of the epidermal growth factor receptor and concurrent activation of the mitogen-activated protein kinase signaling pathway (15). Recent studies have shown that decorin binds to the IGFR-I, inducing its phosphorylation and activation, followed by receptor down-regulation (13). The use of a specific inhibitor to this kinase activity did.
(9) LRP-1-decorin Are Required for TGF- Signaling. 18850 JOURNAL OF BIOLOGICAL CHEMISTRY. 28 –33 43. Ishitobi, M., Haginoya, K., Zhao, Y., Ohnuma, A., Minato, J., Yanagisawa, T., Tanabu, M., Kikuchi, M., and Iinuma, K. (2000) Neuroreport 11, 4033– 4035 44. Watanabe, H., de Caestecker, M. P., and Yamada, Y. (2001) J. Biol. Chem. 276, 14466 –14473 45. Matsumoto-Ida, M., Takimoto, Y., Aoyama, T., Akao, M., Takeda, T., and Kita, T. (2006) Am. J. Physiol. 290, H709 –H715 46. Wilkes, M. C., Mitchell, H., Penheiter, S. G., Dore, J. J., Suzuki, K., Edens, M., Sharma, D. K., Pagano, R. E., and Leof, E. B. (2005) Cancer Res. 65, 10431–10440 47. Ohashi, N., Yamamoto, T., Uchida, C., Togawa, A., Fukasawa, H., Fujigaki, Y., Suzuki, S., Kitagawa, K., Hattori, T., Oda, T., Hayashi, H., Hishida, A., and Kitagawa, M. (2005) FEBS Lett. 579, 2557–2563 48. Runyan, C. E., Schnaper, H. W., and Poncelet, A. C. (2004) J. Biol. Chem. 279, 2632–2639 49. Loukinova, E., Ranganathan, S., Kuznetsov, S., Gorlatova, N., Migliorini, M. M., Loukinov, D., Ulery, P. G., Mikhailenko, I., Lawrence, D. A., and Strickland, D. K. (2002) J. Biol. Chem. 277, 15499 –15506 50. Yang, M., Huang, H., Li, J., Li, D., and Wang, H. (2004) FASEB. J. 18, 1920 –1921 51. Huang, S. S., Leal, S. M., Chen, C. L., Liu, I. H., and Huang, J. S. (2004) FASEB. J. 18, 1719 –1721 52. Tseng, W. F., Huang, S. S., and Huang, J. S. (2004) FEBS Lett. 562, 71–78 53. Nili, N., Cheema, A. N., Giordano, F. J., Barolet, A. W., Babaei, S., Hickey, R., Eskandarian, M. R., Smeets, M., Butany, J., Pasterkamp, G., and Strauss, B. H. (2003) Am. J. Pathol. 163, 869 – 878 54. Schonherr, E., Broszat, M., Brandan, E., Bruckner, P., and Kresse, H. (1998) Arch. Biochem. Biophys. 355, 241–248 55. Winnemoller, M., Schon, P., Vischer, P., and Kresse, H. (1992) Eur. J. Cell Biol. 59, 47–55 56. Huang, S. S., Ling, T. Y., Tseng, W. F., Huang, Y. H., Tang, F. M., Leal, S. M., and Huang, J. S. (2003) FASEB. J. 17, 2068 –2081 57. Leal, S. M., Huang, S. S., and Huang, J. S. (1999) J. Biol. Chem. 274, 6711– 6717 58. Brandan, E., Fuentes, M. E., and Andrade, W. (1991) Eur. J. Cell Biol. 55, 209 –216 59. Larrain, J., Alvarez, J., Hassell, J. R., and Brandan, E. (1997) Exp. Cell Res. 234, 405– 412 60. Brandan, E., Carey, D. J., Larrain, J., Melo, F., and Campos, A. (1996) Eur. J. Cell Biol. 71, 170 –176 61. Casar, J. C., McKechnie, B. A., Fallon, J. R., Young, M. F., and Brandan, E. (2004) Dev. Biol. 268, 358 –371. VOLUME 282 • NUMBER 26 • JUNE 29, 2007. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. 17. Yamaguchi, Y., Mann, D., and Ruoslahti, E. (1990) Nature 346, 281–284 18. Riquelme, C., Larrain, J., Schonherr, E., Henriquez, J. P., Kresse, H., and Brandan, E. (2001) J. Biol. Chem. 276, 3589 –3596 19. Bernfield, M., Gotte, M., Park, P. W., Reizes, O., Fitzgerald, M. L., Lincecum, J., and Zako, M. (1999) Annu. Rev. Biochem. 68, 729 –777 20. Iozzo, R. V. (1998) Annu. Rev. Biochem. 67, 609 – 652 21. Hausser, H., Ober, B., Quentin-Hoffmann, E., Schmidt, B., and Kresse, H. (1992) J. Biol. Chem. 267, 11559 –11564 22. Hausser, H., Schonherr, E., Muller, M., Liszio, C., Bin, Z., Fisher, L. W., and Kresse, H. (1998) Arch Biochem. Biophys 349, 363–370 23. Brandan, E., Retamal, C., Cabello-Verrugio, C., and Marzolo, M. P. (2006) J. Biol. Chem. 281, 31562–31571 24. Herz, J., and Strickland, D. K. (2001) J. Clin. Investig. 108, 779 –784 25. Bu, G., and Marzolo, M. P. (2000) Trends Cardiovasc. Med. 10, 148 –155 26. Massague, J., Cheifetz, S., Endo, T., and Nadal-Ginard, B. (1986) Proc. Natl. Acad. Sci. U. S. A. 83, 8206 – 8210 27. Florini, J. R., Roberts, A. B., Ewton, D. Z., Falen, S. L., Flanders, K. C., and Sporn, M. B. (1986) J. Biol. Chem. 261, 16509 –16513 28. Yaffe, D., and Saxel, O. (1977) Nature 270, 725–727 29. Larrain, J., Carey, D. J., and Brandan, E. (1998) J. Biol. Chem. 273, 32288 –32296 30. Droguett, R., Cabello-Verrugio, C., Riquelme, C., and Brandan, E. (2006) Matrix. Biol. 25, 332–341 31. Hjelmeland, M. D., Hjelmeland, A. B., Sathornsumetee, S., Reese, E. D., Herbstreith, M. H., Laping, N. J., Friedman, H. S., Bigner, D. D., Wang, X. F., and Rich, J. N. (2004) Mol. Cancer Ther. 3, 737–745 32. Osses, N., and Brandan, E. (2002) Am. J. Physiol. 282, C383–C394 33. Obermoeller, L. M., Chen, Z., Schwartz, A. L., and Bu, G. (1998) J. Biol. Chem. 273, 22374 –22381 34. Wrana, J. L., Attisano, L., Carcamo, J., Zentella, A., Doody, J., Laiho, M., Wang, X.-F., and Massague, J. (1992) Cell 71, 1003–1014 35. Leask, A., and Abraham, D. J. (2004) FASEB. J. 18, 816 – 827 36. Ungefroren, H., Lenschow, W., Chen, W. B., Faendrich, F., and Kalthoff, H. (2003) J. Biol. Chem. 278, 11041–11049 37. Kresse, H., and Schonherr, E. (2001) J. Cell. Physiol. 189, 266 –274 38. Costacurta, A., Priante, G., D’Angelo, A., Chieco-Bianchi, L., and Cantaro, S. (2002) J. Clin. Lab. Anal. 16, 178 –186 39. Stander, M., Naumann, U., Dumitrescu, L., Heneka, M., Loschmann, P., Gulbins, E., Dichgans, J., and Weller, M. (1998) Gene Ther. 5, 1187–1194 40. Rapraeger, A. C. (2000) J. Cell Biol. 149, 995–998 41. Abdel-Wahab, N., Wicks, S. J., Mason, R. M., and Chantry, A. (2002) Biochem. J. 362, 643– 649 42. Bernasconi, P., Di Blasi, C., Mora, M., Morandi, L., Galbiati, S., Confalonieri, P., Cornelio, F., and Mantegazza, R. (1999) Neuromuscul. Disord. 9,.
(10) A Novel Modulatory Mechanism of Transforming Growth Factor-β Signaling through Decorin and LRP-1 Claudio Cabello-Verrugio and Enrique Brandan J. Biol. Chem. 2007, 282:18842-18850. doi: 10.1074/jbc.M700243200 originally published online May 7, 2007. Access the most updated version of this article at doi: 10.1074/jbc.M700243200. Click here to choose from all of JBC's e-mail alerts. Supplemental material: http://www.jbc.org/content/suppl/2007/05/08/M700243200.DC1 This article cites 61 references, 25 of which can be accessed free at http://www.jbc.org/content/282/26/18842.full.html#ref-list-1. Downloaded from http://www.jbc.org/ at PONTIFICIA UNIVERSIDAD on December 11, 2017. Alerts: • When this article is cited • When a correction for this article is posted.
(11)
Figure
Documento similar
In agreement with the observed changes in hepatic SREBP-1c expression in liver, central leptin blunted the up- regulation of the mRNA levels of ACC, FAS, and SCD-1 elicited by the
cruzi infection and ET-1 cooperatively activated the Ca 2+ / calcineurin (Cn)/nuclear factor of activated T cells (NFAT) signaling pathway in atrial myocytes, leading to COX-2
In this review we will briefly summarize: (i) the sites of production, mechanism of action and signaling pathways that are activated by ROS and (ii) the role of the mitochondrial H
More importantly, whatever were the specific effects of PMEPA1 in the TGF-β signaling pathways, PMEPA1 overexpression induced, whereas its silencing reduced, faster cell growth
Conversely, the evaluation of mRNA levels of genes encoding pro-in flammatory cytokines [interleukin-1 beta (IL-1 b ), tumor necrosis factor alpha (TNF a ), and transforming
Catalase treatment decreases transforming growth factor-β1 (TGF-β1) mRNA and promoter activity, inhibits the binding of AP-1 to TGF-β1 promoter and reduces the 3TP-Lux
To better characterize the molecular basis of this inhibitory effect of TGF  signaling on chondrogenesis, we have analyzed by qPCR changes in the expression of Sox9 and Aggrecan,
The expansionary monetary policy measures have had a negative impact on net interest margins both via the reduction in interest rates and –less powerfully- the flattening of the