Novel lineages of Prochlorococcus thrive within the oxygen minimum zone of the eastern tropical South Pacific
Texto completo
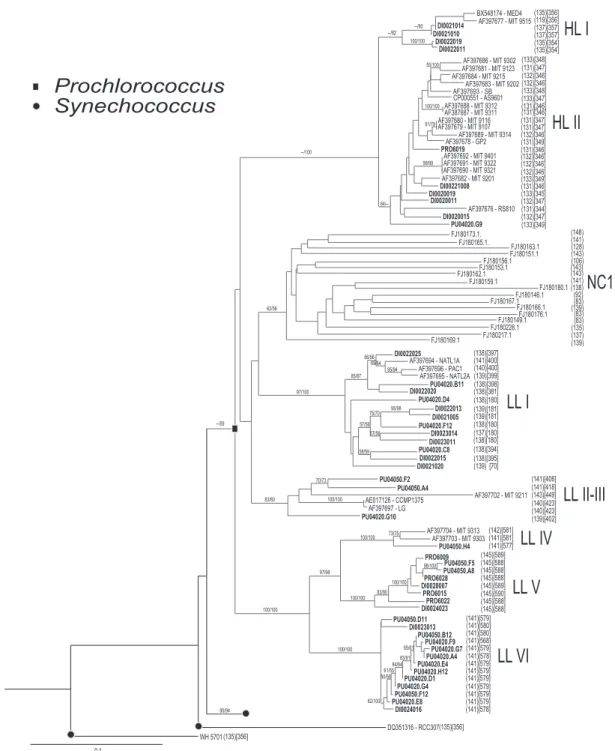
(2) 729 P. Lavin et al. though, a clear geographic horizontal partitioning (Fuller et al., 2003; Zwirglmaier et al., 2007). Moreover, while Prochlorococcus dominates in oligotrophic waters and is absent in eutrophic coastal waters (Olson et al., 1990; Li, 1995; Liu et al., 1997a; Grob et al., 2007), Synechococcus is the dominant phytoplankter in mesotrophic waters, while also inhabiting nutrient-rich coastal waters (Li, 1995; Liu et al., 1998; Grob et al., 2007; Zwirglmaier et al., 2007). The spatial distribution of Prochlorococcus and Synechococcus lineages has been studied at the regional and global scale (Bouman et al., 2006; Johnson et al., 2006; Zwirglmaier et al., 2008). These studies have shown clear distribution patterns associated with environmental conditions, such as light, temperature, nutrients and vertical mixing, which in turn defined current marine biomes and biogeographical provinces (Longhurst, 1998). However, the role of oxygen as a factor influencing the distribution patterns of these microorganisms (and pelagic organisms in general), and even in the definition of marine biogeographical regions, has not been considered. Terminal restriction fragment length polymorphisms (T-RFLP) is a molecular fingerprinting technique that has been widely used in the study of microbial communities (Liu et al., 1997b; Scala and Kerkhof, 2000), including denitrifying bacteria in OMZs (Castro-González et al., 2005). However, until now it has not been applied to biogeographical studies of Prochlorococcus and Synechococcus. Recently, Lavin and colleagues (2008) showed that T-RFLP applied to the ITS region, using just a single endonuclease, can clearly discriminate between HL and LL Prochlorococcus and amongst eight Synechococcus clades. Here, we use cloning and sequencing combined with T-RFLP analysis on the ITS region to determine the genetic diversity and distribution of Prochlorococcus and Synechococcus lineages inhabiting the oxygen-deficient waters of the eastern tropical Pacific.. Results and discussion T-RFLP phylogenetic assignment We analysed 15 T-RFLP profiles of cyanobacterial ITS rRNA sequences from oxic and oxygen-deficient waters of the eastern tropical South Pacific and two profiles from the eastern tropical North Pacific (Fig. S1), obtained during five oceanographic cruises carried out between 2002 and 2006 (Table S1). A database for expected terminal restriction fragments (T-RFs) was used to assign environmental T-RFs to a specific cyanobacterial clade using two restriction enzymes: AluI and BseDI. This database was constructed using 144 sequences from GenBank: 57 from isolated strains and 22 from environ-. mental sequences, as well as 65 new environmental sequences obtained in this work. The final database consisted of 81 ITS sequences that were distinguishable by the combined use of the two restriction enzymes. This approach allowed the discrimination of 19 clades, of which seven belong to Prochlorococcus and include two new ones, termed LLV and LLVI, and the remaining 12 to Synechococcus (Fig. 1). A new Prochlorococcus clade, NC1, recently identified by Martiny and colleagues (2009a), was not included in the current T-RFLP analysis due to a lack of both a BseDI restriction site in the published NC1 sequences and representatives of this lineage in our clone libraries. Moreover, phylogenetic analysis of ITS sequences showed that the NC1 lineage is significantly different from the new clades found in the OMZ (Fig. 1). An average (⫾SD among samples) of 75 (⫾19)% of the T-RFs’ peaks were assigned in our database using the Phylogenetic Analysis Tool (PAT; Kent et al., 2003). They corresponded to 82 (⫾21)% of the total area (an index of abundance) from the environmental T-RFLP profiles. The application of this simple and fast technique allowed the determination of the relative abundance of the majority of the recognized phylotypes studied by dot blot hybridization and qPCR (West and Scanlan, 1999; Ahlgren et al., 2006; Johnson et al., 2006). Moreover, a significant positive correlation was found (Spearman correlation r = 0.6049, P < 0.0001) between the relative abundance of Prochlorococcus and Synechococcus obtained by T-RFLP (Fig. 2A and B) and that obtained by flow cytometry (Fig. 2C and D).. Cyanobacterial diversity and community composition High microdiversity of picocyanobacteria was found along the eastern tropical Pacific by phylogenetic assignment of standardized T-RFLP data. A total of 70 distinct ITS phylotypes, out of the 81 possible ones from our current database, were found in the study region. The number of different ITS sequences detected by T-RFLP in each sample ranged from 3 to 48, with an average of 32 (⫾13). This high richness was consistent with previous studies based on the ITS and RNA-polymerase (rpoC1) genes, obtained by RFLP and cloning and sequencing (Muhling et al., 2005; Haverkamp et al., 2008). A comparison between the cyanobacterial communities of oxic (n = 39) and oxygen-deficient (n = 16) zones showed that OMZ waters presented a greater clade richness and diversity than their oxic counterpart (richness: 5.95 ⫾ 0.42 versus 4.64 ⫾ 0.35, P = 0.04; diversity: 1.07 ⫾ 0.06 versus 0.74 ⫾ 0.07; P = 0.008, respectively, Tukey’s test with a = 0.05). The oxic and suboxic samples showed a variable cyanobacterial composition, with the. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(3) Picocyanobacteria in oxygen minimum zones 730. Fig. 1. Maximum likelihood phylogenetic tree of ITS sequences (using 1100 positions of the alignment, and not including sequences from the tRNAs). Numbers at nodes are support values (bootstrap values) of PhyML and Phylip NJ respectively. The size of T-RFs generated by the endonucleases AluI and BseDI are shown in parentheses and brackets respectively. The 16S rRNA + ITS region from Prochlorococcus and Synechococcus was amplified with primers DINA-F 5′-GAA TCT GCC CTC AGG AGG GGG-3′ and DINA-R 5′-GGG TTG CCC CAT TCG GAA AT-3′ following Lavin and colleagues (2008). The specific primer pair (ITSb-F and DINA-R) for detection and amplification of the ITS region located between the Ala tRNA gene and the 23S rRNA gene were added in the last two cycles of the nested PCR (Lavin et al., 2008). Primer ITSb-F was labelled at the 5′ end with the FAM fluorochrome. The ITS region was sequenced bidirectionally using primer 600f (Laloui et al., 2002) and primers internal to the PCR fragment: DINA-R, ITSb-F (5′-GTT GGT AGA GCG CCT GCT TTG-3′), and NAMO-R (5′-CTC TAA CCA CCT GAG CTA AT-3′). All phylogenetic work was carried out using the Bosque software (Ramírez-Flandes and Ulloa, 2008). Only ITS sequences located between the region flanked by the primer pair 600f and 340r (Laloui et al., 2002) were used and aligned to sequences from NCBI using MUSCLE 3.6 (Edgar, 2004). Phylogenetic trees were inferred by maximum likelihood and distance methods. The maximum likelihood analyses were performed based on the HKY85 model (Hasegawa et al., 1985), using phylogenetic inference based on PhyML (Guindon and Gascuel, 2003). Phylogenetic inferences were also performed with the clustering algorithm (neighbour-joining method) using Phylip (Felsenstein, 2005). Statistical evaluation of tree topologies was performed by bootstrap analysis with 100 resamplings. Environmental sequence data have been deposited in the GenBank database under accession numbers FJ830870 to FJ830934.. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(4) 731 P. Lavin et al.. Fig. 1. cont.. oxic samples less similar to each other than suboxic samples (37.50% and 66.29% similarity using SIMPER respectively, Table 1). The comparison between oxic and suboxic communities showed 68.14% dissimilarity. (Table 1) with significant differences in the composition [two-way crossed analysis of similarity (ANOSIM), R = 0.618, P = 0.006 – Fig. S2]. The presence of Prochlorococcus LLII–III and Synechococcus clade CRD2 in oxic. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
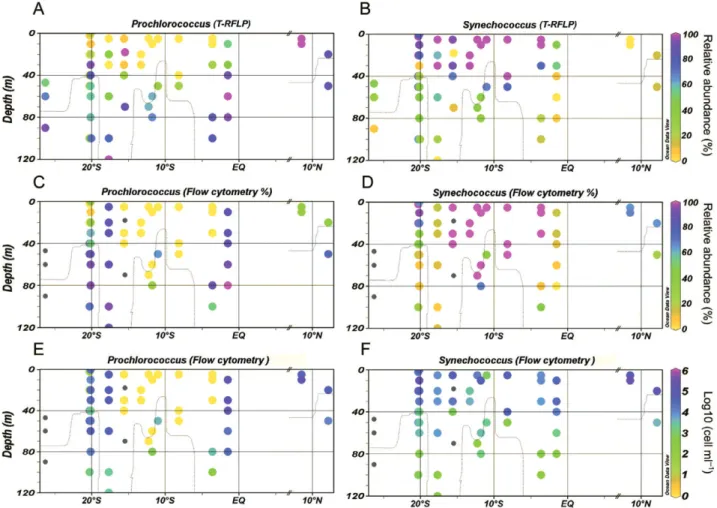
(5) Picocyanobacteria in oxygen minimum zones 732. Fig. 2. Distribution of Prochlorococcus and Synechococcus along the eastern tropical Pacific as determined by T-RFLP (relative abundance, A, B) and flow cytometry (relative abundance, C, D; actual abundance, E, F). Relative abundances were calculated on the basis of the total amount of cyanobacteria (Prochlorococcus plus Synechococcus) per sample. Grey dots represent missing data and the dotted lines the 20 mM O2 isoline (to mark the boundary of the OMZ; Kamykowski and Zentara, 1990) respectively.. samples, and of Prochlorococcus LLIV and the novel lineages LLV and LLVI in suboxic samples underlay the differences found between communities in these two zones (Table 1, Fig. 3). The differences were due primarily to the presence of different Prochlorococcus phylotypes in each community (one-way ANOSIM, R = 0.348, P = 0.001) rather than of Synechococcus phylotypes (one-way ANOSIM, R = 0.067, P > 0.05). The almost-exclusive presence of Prochlorococcus in the OMZ of the Arabian Sea was first reported using flow cytometry and pigment analysis by Johnson and colleagues (1999) and Goericke and colleagues (2000), but no genetic information was provided. On the other hand, Synechococcus cells have been found inhabiting coastal oxygen-deficient environments, such as the Baltic Sea and Chesapeake Bay (Detmer et al., 1993; Crump et al., 2007), but they have not been found to be the dominant cyanobacteria within oceanic OMZs (Goericke et al., 2000; our results). Their lower abundance in this low-light, low-oxygen zone may be partially explained by their. requirements of higher light intensities for growth than Prochlorococcus (Scanlan and West, 2002). However, other factors such as grazing (Cuevas and Morales, 2006) may also be important in determining the lower relative abundance of Synechococcus in these low oxygen waters. The abundance of Prochlorococcus within the OMZ, as determined by flow cytometry, was between nondetectable and ~45 000 cells per millilitre (Fig. 2F; Fig. S3), representing up to 5.3% of the total microbial prokaryotic community. Prochlorococcus clades showed considerable variation in their relative abundance along the latitudinal profiles but displayed the characteristic niche-partitioning in the water column, with higher abundances of HL-adapted ecotypes in the upper water column, and the dominance of LL-adapted ecotypes at depth (Fig. S4). For Synechococcus, except for clade III, all clades had representatives in both surface oxic waters and deeper low-oxygen waters. However, the relative abundance of. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(6) (2.88) (2.74) (3.35) (1.69) (2.18). (0.93) (1.17) (0.82) (3.35) (2.74) (2.88) (1.69) (2.18) (2.68) (1.92). 4.62 2.98 2.89 1.64 1.66. 0.37 0.57 0.34 2.89 2.98 4.62 1.64 1.66 1.08 1.26. Average abundance (⫾SD) oxic. 4.18 4.01 3.75 0.99 0.86 4.71 2.79 0.26 0.35 0.21. 4.71 4.18 4.01 3.75 2.79 (1.73) (1.60) (1.35) (1.97) (1.04) (1.45) (1.64) (0.60) (0.84) (0.57). (1.45) (1.73) (1.60) (1.35) (1.64). Average abundance (⫾SD) suboxic. 16.46 13.75 13.12 12.71 7.70. 15.26 7.13 5.40 3.41 2.67 (4.09) (2.10) (2.04) (2.20) (1.29). (1.11) (0.71) (0.50) (0.61) (0.46). Average similarity (⫾SD). 9.32 8.56 8.26 7.12 6.53 6.50 5.12 3.92 3.36 2.88. (1.98) (1.96) (2.28) (0.96) (1.12) (1.29) (1.35) (0.79) (0.47) (0.75). Average dissimilarity (⫾SD). 13.68 12.56 12.12 10.45 9.58 9.54 7.51 5.76 4.93 4.23. 24.83 20.75 19.79 19.17 11.62. 40.68 19.00 14.39 9.09 7.12. Contribution (%). 13.68 26.24 38.36 48.81 58.39 67.93 75.44 81.2 86.12 90.35. 24.83 45.58 65.36 84.54 96.16. 40.68 59.68 74.06 83.15 90.28. Cumulative contribution (%). Pro, Prochlorococcus; Syn, Synechococcus. The assigned T-RF’s data were analysed with the multivariate statistical software Primer 6 (Primer-E Ltd, Plymouth, UK). A similarity matrix was calculated using the Bray-Curtis coefficient (Rees et al., 2004). Average abundance was calculated as the average of the relative abundance percentages from the total fluorescence of each T-RFLP profile. Similarity and dissimilarity values were calculated using the similarity index of Bray-Curtis. (Resemblance: S17 Bray Curtis similarity and SIMPER, cut-off 90%). The ‘average similarity/dissimilarity’ values correspond to the intra-group (columns) and between group’s (rows) comparisons. The ‘contribution’ refers to the contribution that each taxon has (based on relative abundances) in the comparison of similarities/dissimilarities.. Oxic samples: average similarity = 37.50 Syn Clade I Syn Clade CRD2 Pro LL II–III Syn Clade VI Pro LL I Suboxic samples: average similarity = 66.29 Syn Clade I Pro LL V Pro LL IV Pro LL VI Syn Clade VI Oxic and suboxic: average dissimilarity = 68.14 Pro LL V Pro LL IV Pro LL VI Pro LL II–III Syn Clade CRD2 Syn Clade I Syn Clade VI Pro LL I Pro HL II Syn Clade V. Clades. Table 1. Average abundance, similarity and dissimilarity (in %) and species contributions among cyanobacterial groups from oxic (> 20 mM O2) and suboxic (ⱕ 20 mM O2) zones.. 733 P. Lavin et al.. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(7) Picocyanobacteria in oxygen minimum zones 734. Fig. 3. Representative profile in the OMZ of the eastern tropical South Pacific (Dinamo Cruise, Table S1) showing the characteristic distributions of (A) chlorophyll fluorescence, and dissolved oxygen, nitrate and nitrite concentrations, (B) cyanobacterial concentration, and relative abundance of specific phylotypes of (C) Prochlorococcus and (D) Synechococcus obtained by T-RFLP. The relative abundance of the T-RFs was determined by calculating the area of each peak as a percentage of the sum of all areas in each sample. Then, the relative abundance of each clade was computed as the percentage of the sum of all the relative abundances of the T-RFs assigned to each particular clade per genus.. Synechococcus was always lower than that of Prochlorococcus within the OMZ, in agreement with our flow cytometry results (Fig. 2) and those of Goericke and colleagues (2000) for the OMZs of eastern tropical North Pacific off Mexico and Arabian Sea. At all stations (Fig. S5), except for Station st-39 in the tropical North Pacific, clade I was the most abundant lineage found within Synechococcus (50.5%), followed by clades CRD2 (21.6%), VI (12.5%), V (6.8%), II (3.8%), IV (2%), XV (1.5%), CRD1 (0.5%), XVI (0.5%), VII (0.2%) and III (0.1%). The presence of clades I and IV in some surface samples in the study region suggests these clades are not confined to latitudes above 30°N and below 30°S (Zwirglmaier et al., 2008). However, their presence along the eastern tropical South Pacific could be explained as a result of the Humboldt (or Peru–. Chile) Current, which transports cold waters equatorward. The near-absence of clade III is consistent with other studies that suggest that this lineage is restricted to warmer oligotrophic waters (Fuller et al., 2005; Zwirglmaier et al., 2008). The dominance of Prochlorococcus over Synechococcus in the OMZ zone, and particularly of LLIV, LLV and LLVI, which together contributed almost 90% of the total Prochlorococcus abundance, is presumably a result of specific physiological adaptation to the prevailing light and nutrient conditions within the OMZ. Even though few Prochlorococcus ecotypes are able to utilize oxidized forms of nitrogen, particularly nitrite (Moore et al., 2002), the biological processes of nitrate and nitrite reduction carried out by the local OMZ microbial community (Farías. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(8) 735 P. Lavin et al. et al., 2007) could support their dominant presence. The possibility also exists that members of the new OMZ clades are able to utilize nitrate, as was recently proposed for natural Prochlorococcus populations in the deep chlorophyll maximum of the Sargasso Sea (Casey et al., 2007), and supported by the widespread occurrence of a genomic island containing nitrite and nitrate assimilation genes in uncultured Prochlorococcus cells from marine waters (Martiny et al., 2009b). While there is no physiological or genomic information yet for the new OMZ lineages, members from LLIV have been described to be particularly abundant in deeper subtropical and tropical waters (Johnson et al., 2006), reflecting their photosynthetic capacity that enable them to have a high growth rate at low photon flux densities of < 20 mmol quanta m-2 s-1 (Moore and Chisholm, 1999). In addition, previous environmental reports (Zinser et al., 2007) showed high abundance of this clade at relatively low temperatures (13–14°C), similar to those found in the OMZs, and corresponding to a few degrees below the limit for growth of the ‘type strain’ MIT9313 (Zinser et al., 2007). The presence of a high microdiversity and multiple phylotypes of picocyanobacteria, particularly of Prochlorococcus, in this environment could reflect interaction and competition for resources with other microorganisms also present, such as nitrifiers and denitrifiers or other bacteria and protists (Zubkov et al., 2003; García-Fernández et al., 2004; Michelou et al., 2007; Mary et al., 2008). Several other factors, such as differences in cell size, differences in light absorption and utilization at low light levels, predatory pressure, and viral control could also be influencing the diversity and composition of picocyanobacteria in the OMZ. In summary, the screening of environmental ITS sequences with T-RFLP allowed assessment of the genetic diversity and relative abundance of picocyanobacteria in OMZs at high-resolution level. With this approach, it was found that new clades of Prochlorococcus dominated the oxygen-deficient waters of the eastern tropical South Pacific. The environmental conditions of oxygen deficiency in OMZs that could be affecting enzymatic processes of competitors such as eukaryotic cells (Goericke et al., 2000) should leave a vacant niche that can be occupied by picocyanobacteria. The dominance of specific clades of Prochlorococcus, and particularly of novel ones, most likely reflects evolutionary adaptations to these particular conditions.. Acknowledgements We thank Gadiel Alarcón for sample collection and Rodrigo De la Iglesia for advice on statistical analyses. We also thank the crew and officers onboard R/V Carlos Porter, R/V Abate. Molina, R/V Knorr and R/V Vaedderen, as well as the chief scientists of the different oceanographic cruises: Giovanni Daneri (CHUPS, PRODEPLOY), James Moffet (PEZ05), Bo Thamdrup (Galathea 3). This work was supported by the Agouron Institute (Grant AIMME1.05), the Millennium Scientific Initiative (MSI, Grant P/04-007-F), and the Chilean National Commission for Scientific and Technological Research (CONICYT) through the FONDAP program (Grants 1501-0007/COPAS Program-2 and 1501-0001/CASEB Program-7). P.L. and J.F.S. were supported by graduate fellowships from CONICYT and P.L. received additional support from the MSI. D.J.S. acknowledges support from the Natural Environment Research Council.. References Ahlgren, N.A., and Rocap, G. (2006) Culture isolation and culture-independent clone libraries reveal new marine Synechococcus ecotypes with distinctive light and N physiologies. Appl Environ Microbiol 72: 7193–7204. Ahlgren, N.A., Rocap, G., and Chisholm, S.W. (2006) Measurement of Prochlorococcus ecotypes using real-time polymerase chain reaction reveals different abundances of genotypes with similar light physiologies. Environ Microbiol 8: 441–454. Bouman, H.A., Ulloa, O., Scanlan, D.J., Zwirglmaier, K., Li, W.K.W., Platt, T., et al. (2006) Oceanographic basis of the global surface distribution of Prochlorococcus ecotypes. Science 312: 918–921. Casey, J.R., Lomas, M.W., Mandecki, J., and Walker, D.E. (2007) Prochlorococcus contributes to new production in the Sargasso Sea deep chlorophyll maximum. Geophys Res Lett 34: 1–5. Castro-González, M., Braker, G., Farías, L., and Ulloa, O. (2005) Communities of nirS-type denitrifiers in the water column of the oxygen minimum zone in the eastern South Pacific. Environ Microbiol 7: 1298–1306. Crump, B.C., Peranteau, C., Beckingham, B., and Cornwell, J.C. (2007) Respiratory succession and community succession of bacterioplankton in seasonally anoxic estuarine waters. Appl Environ Microbiol 73: 6802–6810. Cuevas, L.A., and Morales, C.E. (2006) Nanoheterotroph grazing on bacteria and cyanobacteria in oxic and suboxic waters in coastal upwelling areas off northern Chile. J Plankton Res 28: 385–397. Detmer, A.E., Giesenhagen, H.C., Trenkel, V.M., Auf dem Venne, H., and Jochem, F.J. (1993) Phototrophic and heterotrophic pico-plankton and nanoplankton in anoxic depths of the central Baltic Sea. Mar Ecol Prog Ser 99: 197–203. Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. Escribano, R., Hidalgo, P., and Krautz, C. (2009) Zooplankton associated with the oxygen minimum zone system in the northern upwelling region of Chile during March 2000. Deep Sea Res Part II 56: 1083–1094. Farías, L., Paulmier, A., and Gallegos, M. (2007) Nitrous oxide and N-nutrient cycling in the oxygen minimum zone off northern Chile. Deep Sea Res Part I 54: 164–180. Felsenstein, J. (2005) PHYLIP (Phylogeny Inference. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(9) Picocyanobacteria in oxygen minimum zones 736 Package) Version 3.6. Seattle, WA, USA: Department of Genetics, University of Washington. Fuller, N.J., Marie, D., Partensky, F., Vaulot, D., Post, A.F., and Scanlan, D.J. (2003) Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl Environ Microbiol 69: 2430– 2443. Fuller, N.J., West, N.J., Marie, D., Yallop, M., Rivlin, T., Post, A.F., and Scanlan, D.J. (2005) Dynamics of community structure and phosphate status of picocyanobacterial populations in the Gulf of Aqaba, Red Sea. Limnol Oceanogr 50: 363–375. Galán, A., Molina, V., Thamdruo, B., Woebken, D., Lavik, G., Kuypers, M.M.M., and Ulloa, O. (2009) Anammox bacteria and anaerobic oxidation of ammonium in the oxygen minimum zone off northern Chile. Deep Sea Res Part II 56: 1021–1031. García-Fernández, J.M., Tandeau de Marsac, N., and Diez, J. (2004) Streamlined regulation and gene loss as adaptive mechanisms in Prochlorococcus for optimized nitrogen utilization in oligotrophic environments. Microbiol Mol Biol Rev 68: 630–638. Goericke, R., Olson, R.J., and Shalapyonok, A. (2000) A novel niche for Prochlorococcus sp in low-light suboxic environments in the Arabian Sea and the Eastern Tropical North Pacific. Deep Sea Res Part I 47: 1183–1205. Grob, C., Ulloa, O., Li, W.K.W., Alarcón, G., Fukasawa, M., and Watanabe, S. (2007) Picoplankton abundance and biomass across the eastern South Pacific Ocean along latitude 32.5 degrees S. Mar Ecol Prog Ser 332: 53–62. Guindon, S., and Gascuel, O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704. Hasegawa, M., Kishino, H., and Yano, T. (1985) Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Biol 22: 160–174. Haverkamp, T., Acinas, S.G., Doeleman, M., Stomp, M., Huisman, J., and Stal, L.J. (2008) Diversity and phylogeny of Baltic Sea picocyanobacteria inferred from their ITS and phycobiliprotein operons. Environ Microbiol 10: 174–188. Johnson, Z., Landry, M.L., Bidigare, R.R., Brown, S.L., Campbell, L., Gunderson, J., et al. (1999) Energetics and growth kinetics of a deep Prochlorococcus spp. population in the Arabian Sea. Deep Sea Res Part II 46: 1719–1743. Johnson, Z.I., Zinser, E.R., Coe, A., McNulty, N.P., Woodward, E.M.S., and Chisholm, S.W. (2006) Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311: 1737–1740. Kamykowski, D., and Zentara, S.J. (1990) Hypoxia in the world ocean as recorded in the historical data set. Deep Sea Res Part I 37: 1861–1874. Kent, A.D., Smith, D.J., Benson, B.J., and Triplett, E.W. (2003) Web-based phylogenetic assignment tool for analysis of terminal restriction fragment length polymorphism profiles of microbial communities. Appl Environ Microbiol 69: 6768–6776. Laloui, W., Palinska, K.A., Rippka, R., Partensky, F., de Marsac, N.T., Herdman, M., and Iteman, I. (2002) Genotyping of axenic and non-axenic isolates of the genus Prochlorococcus and the OMF-‘Synechococcus’ clade by. size, sequence analysis or RFLP of the internal transcribed spacer of the ribosomal operon. Microbiology 148: 453– 465. Lavin, P., Gómez, P., González, B., and Ulloa, O. (2008) Diversity of the marine picocyanobacteria Prochlorococcus and Synechococcus assessed by terminal restriction fragment length polymorphisms of 16S-23S rRNA internal transcribed spacer sequences. Rev Chil Hist Nat 81: 515–531. Levin, L. (2003) Oxygen minimum zone benthos: adaptations and community responses to hypoxia. Oceanogr Mar Biol Annu Rev 41: 1–45. Li, W.K.W. (1995) Composition of ultraphytoplankton in the Central North-Atlantic. Mar Ecol Prog Ser 122: 1–8. Liu, H., Campbell, L., Landry, M.R., Nolla, H.A., Brown, S.L., and Constantinou, J. (1998) Prochlorococcus and Synechococcus growth rates and contributions to production in the Arabian Sea during the 1995 Southwest and Northeast Monsoons. Deep Sea Res. Part II 45: 2327–2352. Liu, H.B., Nolla, H.A., and Campbell, L. (1997a) Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat Microb Ecol 12: 39–47. Liu, W.T., Marsh, T.L., Cheng, H., and Forney, L.J. (1997b) Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol 63: 4516– 4522. Longhurst, A. (1998) Ecological Geography of the Sea. San Diego, CA, USA: Academic Press. Martiny, A.C., Tai, A.P., Veneziano, D., Primeau, F., and Chisholm, S.W. (2009a) Taxonomic resolution, ecotypes and the biogeography of Prochlorococcus. Environ Microbiol. 11: 823–832. Martiny, A.C., Kathuria, S., and Berube, P.M. (2009b) Widespread metabolic potential for nitrite and nitrate assimilation among Prochlorococcus ecotypes. Proc Natl Acad Sci USA 106: 10787–10792. Mary, I., Tarran, G.A., Warwick, P.E., Terry, M.J., Scanlan, D.J., Burkill, P.H., and Zubkov, M.V. (2008) Light enhanced amino acid uptake by dominant bacterioplankton groups in surface waters of the Atlantic Ocean. FEMS Microbiol Ecol 63: 36–45. Michelou, V.K., Cottrell, M.T., and Kirchman, D.L. (2007) Light-stimulated bacterial production and amino acid assimilation by cyanobacteria and other microbes in the North Atlantic Ocean. Appl Environ Microbiol 73: 5539– 5546. Moore, L.R., and Chisholm, S.W. (1999) Photophysiology of the marine cyanobacterium Prochlorococcus: Ecotypic differences among cultured isolates. Limnol Oceanogr 44: 628–638. Moore, L.R., Rocap, G., and Chisholm, S.W. (1998) Physiology and molecular phylogeny of co-existing Prochlorococcus ecotypes. Nature 393: 464–467. Moore, L.R., Post, A.F., Rocap, G., and Chisholm, S.W. (2002) Utilization of different nitrogen sources by the marine cyanobacteria Prochlorococcus and Synechococcus. Limnol Oceanogr 47: 989–996. Muhling, M., Fuller, N.J., Millard, A., Somerfield, P.J., Marie, D., Wilson, W.H., et al. (2005) Genetic diversity of marine Synechococcus and co-occurring cyanophage communi-. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(10) 737 P. Lavin et al. ties: evidence for viral control of phytoplankton. Environ Microbiol 7: 499–508. Olson, R.J., Chisholm, S.W., Zettler, E.R., Altabet, M.A., and Dusenberry, J.A. (1990) Spatial and temporal distributions of prochlorophyte picoplankton in the North-Atlantic Ocean. Deep Sea Res Part I 37: 1033–1051. Palenik, B. (1994) Cyanobacterial community structure as seen from RNA polymerase gene sequence analysis. Appl Environ Microbiol 60: 3212–3219. Ramírez-Flandes, S., and Ulloa, O. (2008) Bosque: integrated phylogenetic analysis software. Bioinformatics 24: 2539–2541. Rees, G.N., Baldwin, D.S., Watson, G.O., Perryman, S., and Nielsen, D.L. (2004) Ordination and significance testing of microbial community composition derived from terminal restriction fragment length polymorphisms: application of multivariate statistics. Antonie Van Leeuwenhoek 86: 339– 347. Rocap, G., Distel, D.L., Waterbury, J.B., and Chisholm, S.W. (2002) Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl Environ Microbiol 68: 1180–1191. Scala, D.J., and Kerkhof, L.J. (2000) Horizontal heterogeneity of denitrifying bacterial communities in marine sediments by terminal restriction fragment length polymorphism analysis. Appl Environ Microbiol 66: 1980– 1986. Scanlan, D.J., and West, N.J. (2002) Molecular ecology of the marine cyanobacterial genera Prochlorococcus and Synechococcus. FEMS Microbiol Ecol 40: 1–12. Scanlan, D.J., Ostrowski, M., Mazard, S., Dufresne, A., Garczarek, L., Hess, W.R., et al. (2009) Ecological genomics of marine picocyanobacteria. Microbiol Mol Biol Rev 73: 249– 299. Stevens, H., and Ulloa, O. (2008) Bacterial diversity in the oxygen minimum zone of the eastern tropical South Pacific. Environ Microbiol 10: 1244–1259. Ulloa, O., Belmar, L., Farías, L., Castro-González, M., Galán, A., Lavín, P., et al. (2006) Microbial communities and their biogeochemical role in the water column of the oxygen minimum zone in the eastern South Pacific. Gayana (Concepción) 70: 83–86. West, N.J., and Scanlan, D.J. (1999) Niche-partitioning of Prochlorococcus populations in a stratified water column in the eastern North Atlantic Ocean. Appl Environ Microbiol 65: 2585–2591. Zinser, E.R., Coe, A., Johnson, Z.I., Martiny, A.C., Fuller, N.J., Scanlan, D.J., and Chisholm, S.W. (2006) Prochlorococcus ecotype abundances in the North Atlantic Ocean as revealed by an improved quantitative PCR method. Appl Environ Microbiol 72: 723–732. Zinser, E.R., Johnson, Z.I., Coe, A., Karaca, E., Veneziano, D., and Chisholm, S.W. (2007) Influence of light and temperature on Prochlorococcus ecotype distributions in the Atlantic Ocean. Limnol Oceanogr 52: 2205–2220. Zubkov, M.V., Fuchs, B.M., Tarran, G.A., Burkill, P.H., and Amann, R. (2003) High rate of uptake of organic nitrogen compounds by Prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Appl Environ Microbiol 69: 1299–1304.. Zwirglmaier, K., Heywood, J.L., Chamberlain, K., Woodward, E.M.S., Zubkov, M.V., and Scanlan, D.J. (2007) Basin scale distribution patterns of picocyanobacterial lineages in the Atlantic Ocean. Environ Microbiol 9: 1278–1290. Zwirglmaier, K., Jardillier, L., Ostrowski, M., Mazard, S., Garczarek, L., Vaulot, D., et al. (2008) Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages amongst oceanic biomes. Environ Microbiol 10: 147–161.. Supporting information Additional Supporting Information may be found in the online version of this article: Fig. S1. A. Location of sampling stations along the eastern tropical Pacific Ocean during five cruises (Table S1). B. Composite contour plot of dissolved oxygen concentration (in mM) along the eastern tropical Pacific Ocean. Black dots represent sample depths. A total of 55 seawater (~10 l) samples were collected for environmental DNA isolation from 17 stations. The description of the methods used for the physical and chemical characterization of the water column and collection of DNA samples are given in Galán et al. (2009). In order to interpret biological data in relation to the physical and biogeochemical conditions of the water column, we only used data above the depth of 0.1% of surface light intensity. The depth corresponding to 0.1% of surface light intensity was determined using PAR and SPAR light sensors (Sea-Bird SBE 9), when available, or using chlorophyll-a data as described in Grob et al. (2007). Fig. S2. Two-dimensional non-metric MDS ordination illustrating cyanobacterial clade community structure of oxic (> 20 mM O2) and suboxic (ⱕ 20 mM O2) samples, as defined by Bray–Curtis distances. A global R value higher than 0.5 indicates high differences between groups. Raw datasets consisted of the size of terminal restriction fragments (T-RFs), measured in base pairs (bp), and the area of each peak, measured in fluorescence units (an index of abundance). T-RFs from 50 to 600 bp were included in the analysis, whereas those representing less than 0.5% of the total area were excluded. Data were standardized by calculating the area of each peak as a percentage of the total area. The standardized data were phylogenetically assigned through the use of the web-based resource PAT, which in turn predicts T-RF sizes from a database generated from in silico analysis of known sequences (Kent et al., 2003). After the assignment, the relative abundance of each clade was calculated as the sum of all the relative abundances of the T-RFs assigned to that group. Richness (R) was calculated as the total number of clades per sample. Diversity was described with the Shannon–Weaver index (H′), computed from H′ = S pi*ln(pi), where pi is the relative abundance of each clade. T-test was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California, USA). The assigned T-RF’s data were analysed with the multivariate statistical software Primer 6 (Primer-E Ltd, Plymouth, UK). A similarity matrix was calculated using the Bray-Curtis coefficient (Rees et al., 2004). Non-metric multidimensional scaling (NMDS) analysis was used to group data according to their similarity. One-way and two-way crossed analysis of. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(11) Picocyanobacteria in oxygen minimum zones 738 similarity (ANOSIM) was carried out to examine the statistical significance of grouping (Clarke, 1993). The output statistic, R, takes a value of 0 if there is no separation of community structure due to the factor analysed (in this case oxygen) and 1 if a total separation takes place (Clarke, 1993). Values higher than 0.5 account for high differences, whereas differences between 0.25 and 0.5 are considered as moderate. A similarity of percentages (SIMPER) test was used to calculate the average contribution of individual cyanobacterial clades to the average similarity and dissimilarity among groups. Fig. S3. Representative OMZ profiles showing the characteristic distribution of oxygen concentration, cyanobacterial and prokaryotic abundance obtained by flow cytometry (A, B) and total contribution to total prokaryotic plankton (C, D); (A, C) – Dinamo profile and (B, D) – Prodeploy profile (see Table S1). Fig. S4. Distribution of the relative abundance of Prochlorococcus ecotypes along the eastern tropical Pacific as. determined by T-RFLP. Grey dots represent samples where a particular ecotype was not detected. Dotted lines represent the 20 mM O2 isoline (to mark the boundary of the OMZ; Kamykowski and Zentara, 1990). Fig. S5. Distribution of the relative abundance of Synechococcus clades along the eastern tropical Pacific as determined by T-RFLP. Grey dots represent samples where a particular clade was not detected. Dotted lines represent the 20 mM O2 isoline (to mark the boundary of the OMZ; Kamykowski and Zentara, 1990). Table S1. Sampling location and light and dissolved oxygen conditions during five cruises along the eastern tropical Pacific. Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.. © 2010 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology Reports, 2, 728–738.
(12) Copyright of Environmental Microbiology Reports is the property of Wiley-Blackwell and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use..
(13)
Figure
Documento similar
In the preparation of this report, the Venice Commission has relied on the comments of its rapporteurs; its recently adopted Report on Respect for Democracy, Human Rights and the Rule
H I is the incident wave height, T z is the mean wave period, Ir is the Iribarren number or surf similarity parameter, h is the water depth at the toe of the structure, Ru is the
If the concept of the first digital divide was particularly linked to access to Internet, and the second digital divide to the operational capacity of the ICT‟s, the
1. S., III, 52, 1-3: Examinadas estas cosas por nosotros, sería apropiado a los lugares antes citados tratar lo contado en la historia sobre las Amazonas que había antiguamente
Since such powers frequently exist outside the institutional framework, and/or exercise their influence through channels exempt (or simply out of reach) from any political
In the previous sections we have shown how astronomical alignments and solar hierophanies – with a common interest in the solstices − were substantiated in the
While Russian nostalgia for the late-socialism of the Brezhnev era began only after the clear-cut rupture of 1991, nostalgia for the 1970s seems to have emerged in Algeria
Díaz Soto has raised the point about banning religious garb in the ―public space.‖ He states, ―for example, in most Spanish public Universities, there is a Catholic chapel