Tolerance strategies of Atriplex atacamensis and A halimus in response to multiple abiotic stresses
Texto completo
(2) Pontificia Universidad Católica de Chile Facultad de Agronomía e Ingeniería Forestal. Tolerance strategies of Atriplex atacamensis and A. halimus in response to multiple abiotic stresses. Fabiola Alejandra Orrego Márquez. Thesis to obtain the degree of. Doctor en Ciencias de la Agricultura. Santiago, Chile, April 2019 2.
(3) Thesis presented as part of the requirements for the degree of Doctor in Ciencias de la Agricultura, approved by the. Thesis Committee. _____________________. Dr. Rosanna Ginocchio, Advisor. _____________________. Dr. Claudia Ortíz-Calderón. _____________________. Dr. José Miguel Fariña. Santiago, April 2019 3.
(4) Financial funding support for Fabiola Orrego and all thesis activities was obtained from CONICYT Doctoral Grant (21141059/2014), Center of Applied Ecology & Sustainability (CAPES) by CONICYT PIA/BASAL FB0002 (2014) and Facultad de Agronomía e Ingeniería Forestal UC.. 4.
(5) Acknowledgements I would like to thank mi thesis advisor, Rosanna Ginocchio for her support and dedication during this process, and for teaching me to take care of myself as much as I care for what I do. To mi co-advisor Claudia, for her precise comments on plant physiology and biochemistry that shed light on my understanding of plants. I thank my family, for accompanying me with curiosity and enthusiasm on each step of my academic path. To my beloved Diego, for his permanent support during this process and for being a man I can look up to. To all my professional and scientific colleagues in the RESUME lab, especially to Luz María de La Fuente, that taught me how to work properly with plants. To every one of my friends in FAIF and FCB, thanks for help me broaden my perspectives, and being allies in this beautiful path of knowledge.. 5.
(6) Contents. General introduction .............................................................................................. 11 Hypothesis and objectives ..................................................................................... 26 Chapter 1 Diversidad de halófitas chilenas: distribución, origen y hábito .............................. 29 Introducción ........................................................................................................... 31 Materiales y métodos ............................................................................................ 33 Resultados ............................................................................................................ 34 Discusión y conclusiones ...................................................................................... 38 Agradecimientos.................................................................................................... 44 Referencias ........................................................................................................... 44 Chapter 2 Effect of single and combined Cu, NaCl and water stress on three Atriplex species with phytostabilization potential ............................................................................. 55 Abstract ................................................................................................................. 56 Introduction............................................................................................................ 57 Materials and methods .......................................................................................... 60 Results .................................................................................................................. 63 Discussion ............................................................................................................. 67 Conclusion............................................................................................................. 75 6.
(7) Acknowledgments ................................................................................................. 76 References ............................................................................................................ 76 Chapter 3 Growth and physiological effects of combined Copper, NaCl and water stresses on Atriplex atacamensis and A. halimus ..................................................................... 87 Abstract ................................................................................................................. 88 Introduction............................................................................................................ 89 Materials and methods .......................................................................................... 91 Results .................................................................................................................. 96 Discussion ........................................................................................................... 104 Conclusion........................................................................................................... 112 Acknowledgments ............................................................................................... 113 References .......................................................................................................... 113 General Conclusions ........................................................................................... 130. 7.
(8) Table Index. Chapter 1 TABLA 1. Distribución de la riqueza de especies halófitas de Chile según su familia. .............................................................................................................................. 36 TABLA 2. Riqueza, hábito y origen de las especies halófitas presentes en Chile según su distribución geográfica ........................................................................... 40 ANEXO 1. Listado de especies halófitas presentes en Chile según la base de datos eHALOPH.............................................................................................................. 48. Chapter 2 Table 1. Shoot length (SL), dry root weight (DRW) and dry shoot weight (DSW) of Atriplex atacamensis (AA), A. halimus (AH) and A. nummularia (AN) seedlings subjected to available Cu, NaCl and a PEG-induced water stress for seven days.86. Chapter 3 Table 1. Root length (cm.) and root and shoot fresh weight (g) of Atriplex atacamensis and A. halimus seedlings exposed for 10 days to single and combined Cu, NaCl and PEG stresses. .............................................................. 125 Table 2. Total chlorophyll and carotenoid content of Atriplex atacamensis and A. halimus subjected to single and combined Cu, NaCl and PEG stresses............. 126 Table 3. Root and shoot water content (WC in %) of A. atacamensis and A. halimus seedlings exposed for 10 days to single and combined Cu, NaCl and PEG stresses ............................................................................................................... 127 Table 4. Sodium and K concentration (in mg∙g-1) in roots and leaves of A. atacamensis and A. halimus seedlings exposed for 10 days to single and combined Cu, NaCl and PEG stresses. .............................................................. 128. 8.
(9) Table 5. Reduced and oxidized glutathione (nmol g FW -1) in leaves and roots of Atriplex atacamensis and A. halimus exposed for 10 days to single and combined Cu, NaCl and PEG stresses.. .............................................................................. 129. 9.
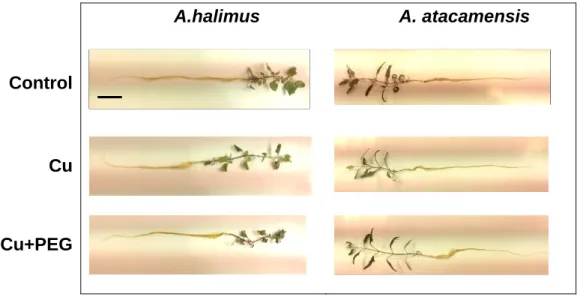
(10) Figure Index. Chapter 2 Figure 1. Root length increase of seedlings of A. atacamensis, A. halimus and A. nummularia subjected to increasing available Cu, NaCl and a decrease of solute potential. ................................................................................................................ 82 Figure 2. Plant water content (g H2O g-1) of Atriplex atacamensis, A. halimus and A. nummularia seedlings subjected to increasing available Cu, NaCl and a decrease of solute potential................................................................................... 83 Figure 3. Increase in root length (cm) of Atriplex atacamensis and A. halimus subjected to increasing single Cu concentrations and its combination with NaCl and PEG. ............................................................................................................... 84 Figure 4. Picture of A. halimus and A. atacamensis seedlings under control, Cu and Cu+PEG treatments of combined stress assay.. ............................................ 85. Chapter 3 Figure 1. Visual symptoms of single and combined stresses on Atriplex seedlings. (a) Loss of turgor and leaf reddening on Atriplex halimus leaves subjected to 7.85 mM PEG. ............................................................................................................. 120 Figure 2. Leaf osmotic potential (MPa) of A. atacamensis (left) and A. halimus (right) seedlings exposed for 10 days to single and combined Cu, NaCl and PEG stresses. .............................................................................................................. 121 Figure 3. Leaf proline (mg g-1 FW) of A. atacamensis (left) and A. halimus (right) seedlings exposed for 10 days to single and combined Cu, NaCl and PEG stresses (n=4; mean± EE). ................................................................................................ 122 Figure 4. Copper concentration (mg∙g-1) in roots and leaves of A. atacamensis (upper) (n=5; means± EE) and A. halimus (lower). ............................................. 123 Figure 5. Non-protein thiols (µmol g-1) in roots and leaves of Atriplex atacamensis (left) and A. halimus (right) seedlings exposed for 10 days to single and combined Cu, NaCl and PEG stresses. ............................................................................... 124. 10.
(11) General Introduction. In Chile there are nearly 550 inactive and abandoned tailing storage facilities (TSF) in the north and central part of the country (Servicio Nacional de Geología y Minería, 2018). Most of these TSF are a byproduct of historical copper production, and as such are not subject to current closure legislation (Servicio nacional de Geología y Minería, 2018). Exposure of abandoned and non-stabilized TSFs to geographic and climatic conditions that predominate in these areas, turns them into pinpoints of copper enrichment into nearby ecosystems, productive land and human settlements (Navarro et al., 2008; Montenegro et al., 2009). Copper is a widespread component in soils, but an increase in its concentration due to anthropogenic activities can cause toxicity symptoms in plants, such as root damage, leaf chlorosis and wilting (Kabata-Pendias, 2010; Brahim and Mohamed, 2011). A widely accepted approach for the rehabilitation of copper-enriched sites is phytostabilization, or the use of metal-tolerant plants to immobilize metals in the rhizosphere (Mendez and Maier, 2008; Alford et al., 2010). Previous approaches to phytostabilization focused on metals as the key limiting factor for plant establishment, lead to the search for metal-tolerant plants (methallophytes) or metal hyperaccumulators in the native component of the geographical area of interest (Ginocchio and Baker, 2004; Orchard et al., 2009; Gonzalez et al., 2010; Tapia et al., 2017). Despite being a valid approach, it is known that successive or simultaneous occurrence of other abiotic limitations besides metal enrichment are. 11.
(12) present in these systems and can further restrict plant survival and establishment. The convergence of geographical and climatic conditions such as high temperature, scarce precipitation (Holmgren et al., 2006) and primary salinization of soils in northern and central Chile (Oyarzún and Oyarzún, 2011; Casanova et al., 2013) can heavily restrict phytostabilization efforts in these ecosystems (Mendez and Maier, 2007; Ginocchio et al., 2017). Under these conditions it is key to consider the simultaneous effects of metals, salt and water stress in plant growth and tolerance traits as a tool to redefine parameters for proper species selection in phytostabilization programs (Mendez and Maier, 2007; Lutts and Lefevre, 2015). Metal, salt and water stresses cause similar restrictions to plant metabolism and growth when studied separately (Saslis-Lagoudakis et al., 2014). An increase in the assimilation of sodium or metal ions within plant cells caused by external salt or metal enrichment can interfere with protein structure and membrane integrity or trigger oxidative damage (Clijsters et al., 1999; Dietz et al., 1999; Reichman, 2002; Zhang et al., 2014; Flowers et al., 2015). This causes a decrease in photosynthetic efficiency, and an overall interruption of plant growth (Munns and Tester, 2008; Stepien and Johnson, 2009). Water stress caused by the osmotic effects of salt assimilation, metal-induced root damage or a decrease in external water supply can also lead to oxidative damage and a decrease in photosynthetic efficiency, which ultimately results in growth impairments or wilting (Moran et al., 1994; Hernández et al., 1995; Chaves et al., 2002). The convergence of the effects of salt, metal and water stresses suggests that plants with a broad response range to these. abiotic conditions. could. represent. a. good. alternative. to. assess. phytostabilization in arid and semiarid regions (Lutts and Lefevre, 2015). Halophyte 12.
(13) species have been identified as good candidates for the rehabilitation of these specific metal enriched sites (Manousaki and Kalogerakis, 2011a). Halophytes are a highly diverse group of species that complete their life cycle in saline habitats with an NaCl concentration of at least 200 mM (Flowers and Colmer, 2008) or 4 dS mol-1 of soil electroconductivity (Koyro, 1999). Even though they only represent 0.2% of all flowering species (Flowers and Colmer, 2015), halophytes derive from a wide range of lineages, are present in all life forms and present a wide range of tolerance mechanisms that allows them to colonize saline ecosystems from deserts to mangroves (Flowers et al., 2010; Manousaki and Kalogerakis, 2011b). One of the main assumptions behind the use of halophyte species for the rehabilitation of metal enriched systems is that halophyte tolerance traits to resist or evade toxicity or osmotic symptoms of salinity can also be effective to bear primary or secondary effects of metal stress (Lutts and Lefevre, 2015; Nikalje and Suprasanna, 2018). Antioxidant, chelating agents and compatible osmolyte synthesis are three tolerance mechanisms present in halophytes that could be specifically used against metal stressors (Figure 1).. 13.
(14) Figure 1. Effect of metal toxicity in plants and avoidance or tolerance responses used by halophytes. Modified from Lutts & Lefevre (2015).. Antioxidant response in halophytes Metal-induced oxidative damage operates through the Fenton reaction, where unpaired metal electrons (Me2+) reduce hydrogen peroxide (H2O2) to form hydroxyl radicals (∙OH) (Seckin Dinler et al., 2010). This reactive oxygen species (ROS) is particularly toxic for plants, because it has the potential to oxidize biomolecules such as lipids, nucleic acids and proteins (Jara-Hermosilla et al., 2017). Halophyte antioxidant response to metals is naturally augmented. due to their. permanent relationship with different salts in their growing habitats (Nagajyoti et al., 2010). However, there are important intraspecific differences regarding antioxidant strategies to overcome an oxidative situation caused by the assimilation of metal ions. For example, Duarte et al., (2013) found that Spartina maritima presents a 14.
(15) rather close relationship between metal concentration and superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPX) and nonenzymatic activity when exposed to a contaminated site (Duarte et al., 2013). A related species, S. densiflora, has a temporal organization of the antioxidant enzymes APX, catalase (CAT), peroxidase (POD) and SOD activity, modulated by H2O2 fluctuations during the oxidative process (Martínez Domínguez et al., 2010). Interestingly, a different response arises when salt and metal stresses are combined. In Suaeda fruticosa, leaf enzymatic activity of APX, CAT and GPX decreases when NaCl is combined with Cu or Cd (Bankaji et al., 2016a), which suggests metal damage to the enzyme structure that undermines its antioxidant ability (Schützendübel and Polle, 2002). Evidence on how the combination of salt and metal stress increases oxidative damage above the antioxidant potential of the plant and how this affects plant functioning, raises questions about oxidative thresholds in halophytes, the effect of metal stressors on antioxidant structure and functioning of halophytes.. Synthesis of chelating agents Synthesis of chelating agents is a critical strategy, triggered when plant tissues reach phytotoxic metal concentrations. Chelating agents combine with ionic forms of metals that normally have the most lethal effects to form inactive complexes (Memon and Schröder, 2009). These complexes are later stored in cell compartments, mainly the cell wall or vacuole. Synthesis of chelating agents is a widespread trait in the plant kingdom, and several molecules are recognized as. 15.
(16) metal chelators (Rauser, 1999); one of the most studied chelating agents related to metal tolerance are phytochelatins. Phytochelatins (PC) are cysteine-rich peptides synthetized from the non-enzymatic antioxidant gluthatione (GSH), which chelate and deactivate several metal components (Grill et al., 1989). Evidence of PC chelation under non-toxic concentrations of metals involved in metabolism, such as Fe, was reviewed early in many sensitive plants (Wagner, 1993; Cobbett, 2000). It has been proposed that the main function of PC is to maintain metal homeostasis in plants, with active chelation and detoxification only in metal stress scenarios (Liu et al., 2015). Phytochelatins are metal specific chelators; hence they are not present during salt stress. However, PC synthesis occurs when halophytes are subjected to metal stress. In Suaeda fructicosa, application of Cd and Cu increases PC concentration with a direct decrease in GSH concentration in roots and leaves (Bankaji et al., 2015). Since GSH is a required substrate for PC synthesis, there is an indirect relationship between metal chelation and non-enzymatic antioxidant potential (Yadav, 2010). For example, an increase of phytochelatin synthase in Atriplex halimus in response to Pb and Cd stress is accompanied by an increase in the expression of gluthatione s-transferase (GST), an enzyme that transports GSH to the site where PC will be synthesized (El-Bakatoushi et al., 2015). Under those circumstances, it is also interesting to explore if PC synthesis decreases the antioxidant potential of halophytes and how this translates into plant growth and metal accumulation.. Compatible osmolyte synthesis 16.
(17) Metal assimilation can alter plant water status at three levels: water uptake, water transport and/or water loss. First, water uptake is limited if metal assimilation alters the morphological integrity of the root; then metal assimilation by the plant can influence the number and size of xylem elements, further limiting water transport to upper organs; finally, metals can limit water loss through stomatal modifications or leaf senescence (Barceló and Poschenrieder, 1990). All these events lead to a state of physiological drought that can either aggravate plant health or induce physiological adaptations that allow the plant to reestablish intracellular osmotic balance. One of the most studied osmotic adjustment mechanisms in water stressed plants is the synthesis of compatible osmolytes. Compatible osmolytes are low weight organic molecules that accumulate in the intracellular space and counteract the osmotic effect of inorganic salts in the medium and maintain protein and membrane stability (Ashraf and Foolad, 2007). One of the most studied compatible osmolytes is proline, a common amino acid in halophytes and sensitive plants (Anjum et al., 2014). Proline synthesis in halophytes subjected to metal stress occurs in response to direct structural damage or derived water uptake deficiency. For example, synthesis of proline and other organic acids in Spartina alterniflora is implicated in root chelation and accumulation of Cd (Min-Wei Chai, 2012). In Aeluropus litoralis, proline concentration increases with increasing concentrations of Co, Cd, Au and Pb (Rastgoo and Alemzadeh, 2011). In contrast, proline synthesis of Atriplex halimus subsp. schweinfurthii seems to occur in response to a decrease in root. 17.
(18) water conductivity and tissue water content, instead of metal assimilation (Nedjimi and Daoud, 2009). As can be seen, proline is an essential component of plant abiotic tolerance, because it is present in multiple stress situations (Ashraf and Foolad, 2007). However, its specific behavior during single and multiple stresses remains poorly explored.. Selection of halophyte candidates Species selection is crucial in phytostabilization strategies. Native or endemic species of metal-enriched sites are a priority group, because they are well adapted to local conditions, (Mendez and Maier, 2008; Heckenroth et al., 2016; Ginocchio et al., 2017), can. trigger ecological succession processes on disturbed sites. (Tapia et al., 2017) and can even prevent the introduction of non-native species (Testiati et al., 2013) into rehabilitation sites. However, knowledge about halophyte species in the native component of Chilean flora is scarce, so it is necessary to explore which local species could represent good alternatives for phytostabilization strategies. Atriplex is a halophyte genus of endemic, native and introduced shrubs and herbs present in Chile, whose tolerance to specific metals has been previously assessed with promising results. One of these is Atriplex halimus, an introduced species from Europe whose growth parameters, physiological response and tolerance mechanisms have been tested under Cd (Lefevre et al., 2010; Soualem et al., 2014), As (Tapia et al., 2013) Pb (Manousaki and Kalogerakis, 2009) and Zn (Lutts et al., 2004a) stresses. A. nummularia is an Australian species introduced in Chile 18.
(19) during the 1970s because of its foraging potential (Lailhacar et al., 1995; Fernández et al., 2016). Although its tolerance to metal stress has not been thoroughly studied (Jordan et al., 2002; Mok et al., 2013), it is a fast growing plant with high growth potential under saline conditions (De Melo et al., 2016). One native component of the genus Atriplex is A. atacamensis. This shrub is one of the 23 native or endemic Atriplex species described in Chile (Rosas, 1989); it inhabits dry riverbeds and ravines of the Tarapacá and Antofagasta regions (Rosas, 1989; Fernández et al., 2016). The geographical overlap of this species with areas affected by mining (Lam et al., 2016) has led to a few studies that have shown its ability to tolerate arsenic without serious growth impediments, through its accumulation in roots and osmotic adjustment in leaves (Tapia et al., 2013; Vromman et al., 2016a). The three aforementioned Atriplex species are salt-tolerant shrubs of arid and semiarid areas that can also be found near sites affected by mining. Studies have shown that they can tolerate different metals, which indicates some degree of generality in their tolerance strategies to abiotic stressors. However, there are no studies that address species-specific effects of copper enrichment on growth and physiology, or the way in which its combination with water and salt stress may affect their tolerance strategies. In recognition of this knowledge gap, I propose to study growth parameters, antioxidant response, synthesis of chelating agents and compatible osmolyte response of Atriplex atacamensis, A. halimus and A. nummularia when subjected to single and combined conditions of copper, water and salt stress.. 19.
(20) References Alford, É.R., Pilon-Smits, E. a. H., Paschke, M.W., 2010. Metallophytes—a view from the rhizosphere. Plant Soil 337, 33–50. https://doi.org/10.1007/s11104-0100482-3 Anjum, N.A., Aref, I.M., Duarte, A.C., Pereira, E., Ahmad, I., Iqbal, M., 2014. Glutathione and proline can coordinately make plants withstand the joint attack of metal(loid) and salinity stresses. Front. Plant Sci. 5, 2010–2013. https://doi.org/10.3389/fpls.2014.00662 Ashraf, M., Foolad, M.R., 2007. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006 Bankaji, I., Caçador, I., Sleimi, N., 2016. Assessing of tolerance to metallic and saline stresses in the halophyte Suaeda fruticosa: The indicator role of antioxidative enzymes. Ecol. Indic. 64, 297–308. https://doi.org/10.1016/j.ecolind.2016.01.020 Bankaji, I., Caçador, I., Sleimi, N., 2015. Physiological and biochemical responses of Suaeda fruticosa to cadmium and copper stresses: growth, nutrient uptake, antioxidant enzymes, phytochelatin, and glutathione levels. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-015-4414-x Barceló, J., Poschenrieder, C., 1990. Plant water relations as affected by heavy metal stress : A review. J. Plant Nutr. 13, 1–37. Brahim, L., Mohamed, M., 2011. Effects of copper stress on antioxidative enzymes , chlorophyll and protein content in Atriplex halimus. African J. Biotechnol. 10, 10143–10148. https://doi.org/10.5897/AJB10.1804 Casanova, M., Salazar, O., Seguel, O., Luzio, W., 2013. Main features of chilean soils, in: The Soils of Chile. Springer Netherlands, Dordrecht. Chaves, M.M., Pereira, J.S., Maroco, J., Rodrigues, M.L., Ricardo, C.P.., Osório, M.L., Carvalho, I., Faria, T., Pinheiro, C., 2002. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 89, 907–916. https://doi.org/10.1093/aob/mcf105 Clijsters, H., Cuypers, A., Vangronsveld, J., 1999. Physiological responses to heavy metals in higher plants; defence against oxidative stress, in: Zeitschrift Fur Naturforschung - Section C Journal of Biosciences. Cobbett, C.S., 2000. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr. Opin. Plant Biol. https://doi.org/10.1016/S13695266(00)00066-2 De Melo, H.F., De Souza, E.R., De Almeida, B.G., Dos, M.B.G., Freire, S., Maia, F.E., 2016. Growth, biomass production and ions accumulation in Atriplex. 20.
(21) nummularia Lindl grown under abiotic stress. Bras. Eng. Agríc. Ambient. 2020. https://doi.org/10.1590/1807-1929/agriambi.v20n2p144-151 Dietz, K.-J., Baier, M., Kramer, V., 1999. Free Radicals and Reactive Oxygen Species as Mediators of Heavy Metal Toxicity in Plants, in: Heavy Metal Stress in Plants. Duarte, B., Santos, D., Caçador, I., 2013. Halophyte anti-oxidant feedback seasonality in two salt marshes with different degrees of metal contamination: Search for an efficient biomarker. Funct. Plant Biol. 40, 922–930. https://doi.org/10.1071/FP12315 El-Bakatoushi, R., Alframawy, A.M., Tammam, A., Youssef, D., El-Sadek, L., 2015. Molecular and Physiological Mechanisms of Heavy Metal Tolerance in Atriplex halimus. Int. J. Phytoremediation 17, 789–800. https://doi.org/10.1080/15226514.2014.964844 Fernández, Y.T., Diaz, O., Acuña, E., Casanova, M., Salazar, O., Masaguer, A., 2016. Phytostabilization of arsenic in soils with plants of the genus Atriplex established in situ in the Atacama Desert. Environ. Monit. Assess. 188, 235. https://doi.org/10.1007/s10661-016-5247-x Flowers, T.J., Colmer, T.D., 2015. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 115, 327–331. https://doi.org/10.1093/aob/mcu267 Flowers, T.J., Colmer, T.D., 2008. Salinity tolerance in halophytes. New Phyotologist 945–963. Flowers, T.J., Galal, H.K., Bromham, L., 2010. Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct. Plant Biol. 37, 604–612. Flowers, T.J., Munns, R., Colmer, T.D., 2015. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 115, 419–431. https://doi.org/10.1093/aob/mcu217 Ginocchio, R., Baker, A.J.M., 2004. Metallophytes in Latin America: a remarkable biological and genetic resource scarcely known and studied in the region. Rev. Chil. Hist. Nat. 185–194. Ginocchio, R., León-Lobos, P., Arellano, E.C., Anic, V., Ovalle, J.F., Baker, A.J.M., 2017. Soil physicochemical factors as environmental filters for spontaneous plant colonization of abandoned tailing dumps. Environ. Sci. Pollut. Res. 24, 13484– 13496. https://doi.org/10.1007/s11356-017-8894-8 Gonzalez, I., Cisternas, M., Kelm, U., Neaman, A., 2010. Metalofitas en el teniente y su potencial para la remediación de suelos contaminados por cobre. Cienc. Ahora No. 25, 29–35. Grill, E., Löffler, S., Winnacker, E.L., Zenk, M.H., 1989. Phytochelatins, the heavymetal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. U. S. A. https://doi.org/10.1073/pnas.86.18.6838 21.
(22) Heckenroth, A., Rabier, J., Dutoit, T., Torre, F., Prudent, P., Laffont-Schwob, I., 2016. Selection of native plants with phytoremediation potential for highly contaminated Mediterranean soil restoration: Tools for a non-destructive and integrative approach. J. Environ. Manage. 183, 850–863. https://doi.org/https://doi.org/10.1016/j.jenvman.2016.09.029 Hernández, J. a., Olmos, E., Corpas, F.J., Sevilla, F., del Río, L. a., 1995. Saltinduced oxidative stress in chloroplasts of pea plants. Plant Sci. 105, 151–167. https://doi.org/10.1016/0168-9452(94)04047-8 Holmgren, M., Stapp, P., Dickman, C., Gracia, C., Graham, S., Gutiérrez, J., Hice, C., Jaksic, F., Kelt, D., Letnic, M., Lima, M., López, B., Meserve, P., Milstead, B., Polis, G., Previtali, A., Richte, M., Sabaté, S., Squeo, F.A., 2006. Extreme climatic events shape arid and\rsemiarid ecosystems. Front Ecol Env. 4, 87–95. https://doi.org/10.1890/1540-9295(2006)004[0087:ECESAA]2.0.CO;2 Jara-Hermosilla, D., Barros-Vásquez, D., Muñoz-Rojas, A., Castro-Morales, S., Ortiz-Calderón, C., 2017. Enzymatic reduction of hydrogen peroxide on Polypogon australis plants grown in a copper mining liquid waste. South African J. Bot. 109, 42–49. https://doi.org/10.1016/j.sajb.2016.12.017 Jordan, F.L., Robin-Abbott, M., Maier, R.M., Glenn, E.P., 2002. A comparison of chelator-facilitated metal uptake by a halophyte and a glycophyte. Environ. Toxicol. Chem. 21, 2698–704. Kabata-Pendias, A., 2010. Chapter 16. Elements of Group 11, in: Press, C. (Ed.), Trace Elements in Soils and Plants. p. 548. Koyro, H.-W., 1999. Study of potential cash crop halophytes by a quick check system: Determination of the threshold of salinity tolerance and the ecophysiological demands. Lailhacar, S., Hugo, R., Silva, H., Caldentey, J., 1995. Rendimiento de leña y recuperación al corte en diferentes especies y procedencias arbustivas del género Atriplex. Rev. Ciencias For. 10, 85–97. Lam, E.J., Gálvez, M.E., Cánovas, M., Montofré, I.L., Rivero, D., Faz, A., 2016. Evaluation of metal mobility from copper mine tailings in northern Chile. Environ. Sci. Pollut. Res. 23, 11901–11915. https://doi.org/10.1007/s11356-016-6405-y Lefevre, I., Marchal, G., Edmond Ghanem, M., Correal, E., Lutts, S., 2010. Cadmium has contrasting effects on polyethylene glycol - Sensitive and resistant cell lines in the Mediterranean halophyte species Atriplex halimus L. J. Plant Physiol. 167, 365–374. https://doi.org/10.1016/j.jplph.2009.09.019 Liu, W., Zhang, X., Liang, L., Chen, C., Wei, S., 2015. Reactive Oxygen Species and Oxidative Damage in Plants Under Stress. https://doi.org/10.1007/978-3-31920421-5. 22.
(23) Lutts, S., Lefevre, I., 2015. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 509–528. https://doi.org/10.1093/aob/mcu264 Lutts, S., Lefèvre, I., Delpérée, C., Kivits, S., Dechamps, C., Robledo, A., Correal, E., 2004. Heavy Metal Accumulation by the Halophyte Species Mediterranean Saltbush. J. Environ. Qual. 33, 1271. https://doi.org/10.2134/jeq2004.1271 Manousaki, E., Kalogerakis, N., 2011a. Halophytes Present New Opportunities in Phytoremediation of Heavy Metals and Saline Soils. Ind. Eng. Chem. Res. 50, 656–660. https://doi.org/10.1021/ie100270x Manousaki, E., Kalogerakis, N., 2011b. Halophytes—An Emerging Trend in Phytoremediation. Int. J. Phytoremediation 13, 959–969. https://doi.org/10.1080/15226514.2010.532241 Manousaki, E., Kalogerakis, N., 2009. Phytoextraction of Pb and Cd by the Mediterranean saltbush (AtripLex halimus L.): Metal uptake in relation to salinity. Environ. Sci. Pollut. Res. 16, 844–854. https://doi.org/10.1007/s11356-009-0224-3 Martínez Domínguez, D., Córdoba García, F., Canalejo Raya, A., Torronteras Santiago, R., 2010. Cadmium-induced oxidative stress and the response of the antioxidative defense system in Spartina densiflora. Physiol. Plant. https://doi.org/10.1111/j.1399-3054.2010.01368.x Memon, A.R., Schröder, P., 2009. Implications of metal accumulation mechanisms to phytoremediation. Environ. Sci. Pollut. Res. Int. 16, 162–75. https://doi.org/10.1007/s11356-008-0079-z Mendez, M.O., Maier, R.M., 2008. Phytostabilization of mine tailings in arid and semiarid environments--an emerging remediation technology. Environ. Health Perspect. 116, 278–83. https://doi.org/10.1289/ehp.10608 Mendez, M.O., Maier, R.M., 2007. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Bio/Technology 7, 47–59. https://doi.org/10.1007/s11157-007-9125-4 Min-Wei Chai, 2012. Effects of cadmium stress on growth, metal accumulation and organic acids of Spartina alterniflora Loisel. African J. Biotechnol. 11, 6091–6099. https://doi.org/10.5897/AJB11.2804 Mok, H.-F., Majumder, R., Laidlaw, W.S., Gregory, D., Baker, A.J.M., Arndt, S.K., 2013. Native Australian Species are Effective in Extracting Multiple Heavy Metals from Biosolids. Int. J. Phytoremediation 15, 615–632. https://doi.org/10.1080/15226514.2012.723063 Montenegro, G., Fredes, C., Mejías, E., Bonomelli, C., Olivares, L., 2009. Contenidos de metales pesados en suelos cercanos a un relave cuprífero chileno. Agrociencia 43, 427–435. Moran, J.F., Becana, M., Iturbe-ormaetxe, I., Frechilla, S., Klucas, R. V, Apariciotejo, P., Vegetal, D.D.N., Experimental, E., Dei, D.A., Zaragoza, E.-, 1994. Drought 23.
(24) induces oxidative stress in https://doi.org/10.1007/BF00197534. pea. plants.. Planta. 194,. 346–352.. Munns, R., Tester, M., 2008. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 59, 651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911 Nagajyoti, P.C., Lee, K.D., Sreekanth, T.V.M., 2010. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 8, 199–216. https://doi.org/10.1007/s10311-010-0297-8 Navarro, M.C., Pérez-Sirvent, C., Martínez-Sánchez, M.J., Vidal, J., Tovar, P.J., Bech, J., 2008. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochemical Explor. 96, 183–193. https://doi.org/10.1016/j.gexplo.2007.04.011 Nedjimi, B., Daoud, Y., 2009. Cadmium accumulation in Atriplex halimus subsp. schweinfurthii and its influence on growth, proline, root hydraulic conductivity and nutrient uptake. Flora Morphol. Distrib. Funct. Ecol. Plants 204, 316–324. https://doi.org/10.1016/j.flora.2008.03.004 Nikalje, G.C., Suprasanna, P., 2018. Coping With Metal Toxicity – Cues From Halophytes. Front. Plant Sci. 9, 1–11. https://doi.org/10.3389/fpls.2018.00777 Orchard, C., León-Lobos, P., Ginocchio, R., 2009. Phytostabilization of massive mine wastes with native phytogenetic resources: potential for sustainable use and conservation of the native flora in north-central Chile. Cienc. e Investig. Agrar. 36, 329–352. https://doi.org/10.4067/S0718-16202009000300002 Oyarzún, J., Oyarzún, R., 2011. Sustainable development threats, inter-sector conflicts and environmental policy requirements in the arid, mining rich, Northern Chile territory. Sustain. Dev. 19, 263–274. https://doi.org/10.1002/sd.441 Rastgoo, L., Alemzadeh, A., 2011. Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Aust. J. Crop Sci. 5, 375–383. Rauser, W.E., 1999. Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin, and metallothioneins. Cell Biochem. Biophys. 31, 19–48. https://doi.org/10.1007/BF02738153 Reichman, S.M., 2002. The Responses of Plants to Metal Toxicity : A review focusing on Copper , Manganese and Zinc, Environment. Rosas, M.R., 1989. The Genus Atriplex Chenopodiaceae In Chile. Gayana Bot. 46, 3–82. Saslis-Lagoudakis, H.C., Moray, C., Bromham, L., Rajakaruna, N., Boyd, B., Harris, T., 2014. Evolution of Salt Tolerance in Angiosperms: a Phylogenetic Approach. Plant Ecol. Evol. harsh Environ. New York Hauppage 77–95. Schützendübel, A., Polle, A., 2002. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53, 1351–65. 24.
(25) Seckin Dinler, B., Turkan, I., Sekmen, A., Ozfidan-Konakci, C., 2010. The role of antioxidant defense systems at differential salt tolerance of Hordeum marinum Huds. (Sea barley grass) and Hordeum vulgare L. (cultivated barley). Environ. Exp. Bot. 69, 76–85. Servicio nacional de Geología y Minería, 2018. Estudios de normativas internacionales de diseño, construcción, cierre y post cierre de depósitos de relaves. Santiago, Chile. Servicio Nacional de Geología y Minería, 2018. Análisis del Catastro de Depósitos de Relave de Chile. Santiago, Chile. Soualem, S., Adda, A., Belkhodja, M., Merah, O., 2014. Calcium supply reduced effect of salinity on growth in the Mediterranean shrub (Atriplex halimus L.). Life Sci. J. 11, 278–284. Stepien, P., Johnson, G.N., 2009. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 149, 1154– 1165. https://doi.org/10.1104/pp.108.132407 Tapia, Y., Bustos, P., Salazar, O., Casanova, M., Castillo, B., Acuña, E., Masaguer, A., 2017. Phytostabilization of Cu in mine tailings using native plant Carpobrotus aequilaterus and the addition of potassium humates. J. Geochemical Explor. 183, 102–113. https://doi.org/10.1016/j.gexplo.2017.10.008 Tapia, Y., Diaz, O., Pizarro, C., Segura, R., Vines, M., Zúñiga, G., MorenoJiménez, E., 2013. Atriplex atacamensis and Atriplex halimus resist As contamination in Pre-Andean soils (northern Chile). Sci. Total Environ. 450–451, 188–96. https://doi.org/10.1016/j.scitotenv.2013.02.021 Testiati, E., Parinet, J., Massiani, C., Laffont-Schwob, I., Rabier, J., Pfeifer, H.-R., Lenoble, V., Masotti, V., Prudent, P., 2013. Trace metal and metalloid contamination levels in soils and in two native plant species of a former industrial site: Evaluation of the phytostabilization potential. J. Hazard. Mater. 248–249, 131– 141. https://doi.org/https://doi.org/10.1016/j.jhazmat.2012.12.039 Vromman, D., Lefèvre, I., Šlejkovec, Z., Martínez, J.-P.P., Vanhecke, N., Briceño, M., Kumar, M., Lutts, S., 2016. Salinity influences arsenic resistance in the xerohalophyte Atriplex atacamensis Phil. Environ. Exp. Bot. 126, 32–43. https://doi.org/10.1016/j.envexpbot.2016.01.004 Wagner, G.J., 1993. Accumulation of Cadmium in Crop Plants And Its Consequences to Human Health, Advances in Agronomy. Elsevier Masson SAS. https://doi.org/10.1016/S0065-2113(08)60593-3 Yadav, S.K., 2010. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African J. Bot. https://doi.org/10.1016/j.sajb.2009.10.007. 25.
(26) Zhang, L., Ma, H., Chen, T., Pen, J., Yu, S., Zhao, X., 2014. Morphological and Physiological Responses of Cotton (Gossypium hirsutum L.) Plants to Salinity. PLoS One 9, e112807. https://doi.org/10.1371/journal.pone.0112807. 26.
(27) Hypotheses and objectives Hypotheses. Hypothesis 1: Atriplex atacamensis, A. halimus and A. nummularia seedlings have different tolerance thresholds to increasing conditions of copper, salt and water stresses.. Hypothesis 2: Single copper, salt and water stresses have variable effects on growth parameters of Atriplex atacamensis and A. halimus seedlings and induce the expression of chelating, osmoregulative and antioxidant mechanisms. However, the combination of these stresses negatively affects plant growth and expression of these mechanisms.. General objective. To assess tolerance thresholds and tolerance mechanisms in individuals of three halophyte species of the genus Atriplex when exposed to single and combined copper, salt, and water stresses.. 27.
(28) Specific Objectives. 1. To describe the Chilean halophyte biodiversity and to identify candidate species for phytostabilization of chemically (i.e. metal enriched and salinized) degraded soils.. 2. To assess the effect of increasing conditions of salinity, available copper and water stress on growth parameters of A. atacamensis, A. halimus and A. nummularia seedlings.. 3. To assess single and combined effects of copper, salt and water stresses on growth, ion distribution and the expression of antioxidant, compatible osmolytes and chelating agents used by of A. atacamensis and A. halimus individuals.. 28.
(29) Chapter 1. Diversidad de halófitas chilenas: Distribución, origen y hábito. Fabiola Orrego1,3, Luz María de la Fuente1,3; Miguel Gómez2; Rosanna Ginocchio1,3. 1Departamento. de Ecosistemas y Medio Ambiente, Facultad de Agronomía e Ing.. Forestal, Pontificia Universidad Católica de Chile. Avenida Vicuña Mackenna 4860, Santiago, Chile.. 2Departamento. de Ciencias Vegetales, Facultad de Agronomía e Ing. Forestal,. Pontificia Universidad Católica de Chile. Avenida Vicuña Mackenna 4860, Santiago, Chile.. 3Center. of Applied Ecology and Sustainability, Facultad de Ciencias Biológicas, CP 6513677, Universidad Católica de Chile, Santiago, Chile. This chapter was published in Gayana Botanica – Ediciones de la Universidad de Concepción, Chile (75, 2, 555-567) 29.
(30) RESUMEN Las especies halófitas son reconocidas por su capacidad de sobrevivir en ambientes salinos. Sin embargo, esta condición varía enormemente entre los diferentes taxones de plantas halófitas, dificultando su correcta clasificación. Actualmente, la base de datos eHALOPH registra las especies halófitas de todo el mundo bajo un único criterio de salinidad. En este trabajo se presenta el primer listado de halófitas en Chile, elaborado a partir de eHALOPH. Los resultados indican una diversidad de 138 especies, distribuidas en 31 familias. Más del 80% de estas especies son herbáceas y cerca del 55% son exóticas. La mayor riqueza se encontró en las regiones de Coquimbo y Valparaíso, y la menor en el territorio insular. Estos resultados son una primera aproximación a la diversidad de especies halófitas en Chile. Al mismo tiempo, plantean un desafío para avanzar con su reconocimiento con el fin de proponer posibles estrategias para su conservación y uso.. PALABRAS. CLAVE:. Halófita,. diversidad. vegetal,. ecosistemas. salinos,. eHALOPH.. ABSTRACT Halophyte species are recognized for their ability to survive in salty environments. However, this condition greatly varies among plant taxons, difficulting their proper classification. Currently, the eHALOPH database registers halophyte species from 30.
(31) around the world, under a single salinity criterion. In the present study, the first list of halophyte species present in Chile made from eHALOPH is presented. Results indicate a diversity of 138 species, distributed in 31 families. Over 80% of species are herbaceous and nearly 55% are exotic. The largest richness was found in Coquimbo and Valparaíso regions, and the lowest in the insular territory. These results are a first approximation to halophyte diversity in Chile. At the same time, it poses a challenge to advance in their recognition in order to propose strategies for their conservation and use.. Keywords: Halophyte, plant diversity, saline ecosystems, eHALOPH. INTRODUCCIÓN El término halófita (halos= sal; phyta= planta de) se utiliza hace más de 200 años para definir a aquellas especies de plantas que han hecho de los ecosistemas salinos su hábitat (Flowers et al., 1986). Sin embargo, la gran diversidad de ambientes en los que existen y la gran cantidad de estrategias que usan para tolerar o evadir el exceso de salinidad en el sustrato, ha derivado en la proliferación de variadas definiciones (Breckle 1990, Grigore et al. 2010). Esto crea una fuente de incertidumbre a la hora de clasificar una especie como halófita, tanto a nivel regional como global (Grigore et al., 2014). A pesar de ello, en la literatura actual se ha llegado a un consenso con respecto al uso de la concentración de. cloruro de sodio (NaCl) como el parámetro que permite. discriminar a las especies halófitas de aquellas sensibles a la salinidad (Flowers & 31.
(32) Colmer 2008). En base a esta definición, se han creado los primeros listados de especies halófitas a nivel global (Aronson 1989, Menzel & Lieth 2003, Santos et al. 2015). En la actualidad, perturbaciones tales como la degradación y salinización de los suelos devuelven el interés hacia este grupo de especies. Sus principales usos están enfocados en el desarrollo de la agricultura biosalina (Koyro 2003, Toderich et al. 2008), la rehabilitación de suelos degradados y contaminados con residuos mineros (Al-Farrajii & Al-Hilli 1994, Aronson & Le Floc’h 1995, Lutts & Lefevre 2015), la generación de biomasa para forraje (Lailhacar et al., 1995) y como biofiltro de residuos municipales y aguas residuales (Shpigel et al. 2013, Buhmann et al. 2015). A pesar del gran valor de estas especies, su identificación y clasificación sigue siendo un desafío a nivel local y regional. En Chile, el estudio de este grupo se ha realizado a través de la descripción de las especies presentes en hábitat salinos (San Martín et al. 1992, Teillier 1998, Teillier & Becerra 2003, Ramírez & Álvarez 2012, entre otros) y la evaluación de los rasgos fisiológicos y morfológicos que favorecen la tolerancia a la salinidad (Rhodes & Felker 1988, Poblete et al. 1991, Bartels & Dinakar 2013). Dichos resultados indican que nuestro país posee una riqueza importante de especies halófitas; sin embargo, la información sobre la diversidad de estas especies está descrita sólo a nivel local. Por ello, consideramos esencial identificar la diversidad de este grupo de especies a nivel nacional con el fin de generar estrategias de conservación y definir sus potenciales usos. En este contexto, el objetivo de este trabajo es proponer el primer listado de halófitas vasculares presentes en Chile y señalar sus principales parámetros 32.
(33) ecológicos utilizando el listado global de especies eHALOPH.. MATERIALES Y MÉTODOS. LISTADO DE HALÓFITAS PRESENTES EN CHILE Este trabajo utilizó como fuente principal la base de datos eHALOPH. Esta consiste en un registro global de especies halófitas basado en las publicaciones de Aronson (1989) y Menzel & Lieth (2003), que se mantiene en permanente actualización gracias al aporte de investigadores de todo el mundo (Santos et al. 2015). La definición de halófita que sustenta este listado corresponde a aquellas especies capaces de “tolerar una conductividad eléctrica de al menos 7,8 dSm -1 (equivalente a 80 mM de NaCl) durante tiempos significativos de su ciclo de vida” (http://www.sussex.ac.uk/affiliates/halophytes 09/11/17). Este listado es de libre acceso y está publicado en la web http://www.sussex.ac.uk/affiliates/halophytes/. Para elaborar el listado nacional de halófitas, se contrastó el listado eHALOPH (consultado el 13 de diciembre de 2017) con las especies presentes en Chile según el Catálogo de las Plantas Vasculares del Cono Sur (Zuloaga et al., 2008), disponible en la página web http://www2.darwin.edu.ar/. No se consideraron subespecies ni variedades en la elaboración de este listado. Para cada especie halófita presente en Chile, se registró la familia, hábito, origen y distribución regional. Esta información se recopiló a partir del mismo catálogo y el inventario nacional de especies de Chile (MMA 2017). La distribución regional de las especies se presentó en base a 13 regiones 33.
(34) administrativas y tres zonas insulares: Archipiélago de Juan Fernández, Islas Desventuradas e Isla de Pascua. Esta forma de presentación responde a las categorías de distribución utilizada en el Catálogo de Plantas Vasculares del Cono Sur. Adicionalmente, varias publicaciones fueron utilizadas para complementar los datos faltantes (Marticorena & Quezada 1985, Matthei et al. 1995, Ramírez & San Martín 2006, Fuentes et al. 2013).. RESULTADOS. RIQUEZA ESPECÍFICA DE LAS FAMILIAS DE HALÓFITAS EN CHILE El listado de especies halófitas de Chile está compuesto por un total de 138 especies (Anexo 1), distribuidas en 31 familias (Tabla 1). Las familias con mayor riqueza de halófitas son Poaceae con 29 especies, Amaranthaceae con 20, Fabaceae con 11, Asteraceae con 9 y Cyperaceae con 8 (Tabla 1). De las 31 familias registradas, 26 poseen menos de un 5% de representación. Sólo dos familias tienen más de 10% de representación: Poaceae y Amaranthaceae con 21,01% y 14,49%, respectivamente (Tabla 1). Del grupo de las gimnospermas, solo se encontró la familia Ephedraceae, representada por una especie, Ephedra ochreata. Las otras 30 familias pertenecen al grupo de las angiospermas, dentro de las cuales hay 26 familias de dicotiledóneas y 5 familias de monocotiledóneas.. 34.
(35) DISTRIBUCIÓN, ORIGEN Y HÁBITO DE LAS ESPECIES HALÓFITAS DE CHILE La mayor riqueza de especies halófitas se concentra en la zona centro del país, con una disminución progresiva hacia el extremo sur y el territorio insular (Tabla 2). Las regiones de Coquimbo (COQ) y Valparaíso (VAL) tienen la mayor riqueza, con 81 especies de halófitas cada una. Le siguen las regiones del Biobío (BIO) con 74 y la Metropolitana (RME) con 70. Al contrario, las regiones de Aysén (AIS) y de Magallanes y de la Antártica Chilena (MAG) presentan la menor riqueza de especies del territorio continental, con 25 y 37 registros respectivamente. El territorio insular también presenta una baja riqueza de especies halófitas, siendo las islas Desventuradas la zona de menor número de especies en el territorio nacional (6 especies). De las 138 especies de halófitas que hay en Chile, 4 son endémicas (3%), 58 nativas (42%) y 76 introducidas (55%) (Tabla 2). Las cuatro especies de halófitas endémicas registradas son Prosopis tamarugo, Atriplex atacamensis, Atriplex repanda y Hordeum brachyantherum, todas presentes en la zona norte y centro de Chile (Anexo 1). En la zona sur, la única especie endémica registrada corresponde a Hordeum brachyantherum, presente en la región de Magallanes y de la Antártica Chilena. En el territorio insular no se registran especies endémicas.. 35.
(36) TABLA 1. Distribución de la riqueza de especies halófitas de Chile según su familia (n=138). Los resultados se presentan en orden alfabético. / Distribution of chilean halophyte species richness by its family (n=138). Results presented in alphabetical order.. Familia Aizoaceae. Riqueza. %. 6. 4,35. Amaranthaceae. 20. 14,49. Anacardiaceae. 1. 0,72. Apiaceae. 2. 1,45. Araliaceae. 1. 0,72. Asteraceae. 9. 6,52. Boraginaceae. 1. 0,72. Brassicaceae. 5. 3,62. Caryophyllaceae. 2. 1,45. Convolvulaceae. 2. 1,45. Cyperaceae. 8. 5,80. Ephedraceae. 1. 0,72. Euphorbiaceae. 2. 1,45. 11. 7,97. Frankeniaceae. 2. 1,45. Goodeniaceae. 1. 0,72. Juncaceae. 5. 3,62. Juncaginaceae. 2. 1,45. Malvaceae. 2. 1,45. Plantaginaceae. 7. 5,07. Plumbaginaceae. 1. 0,72. 29. 21,01. Polygonaceae. 3. 2,17. Portulacaceae. 1. 0,72. Potamogetonaceae. 3. 2,17. Primulaceae. 2. 1,45. Fabaceae. Poaceae. 36.
(37) Ruppiaceae. 1. 0,72. Solanaceae. 4. 2,90. Typhaceae. 2. 1,45. Verbenaceae. 1. 0,72. Zygophyllaceae. 1. 0,72. Riqueza total. 138. En Chile continental, las regiones del extremo norte (Arica y Parinacota, Tarapacá, Antofagasta y Atacama) apenas superan el 50% de especies halófitas nativas y endémicas (Tabla 2). Esta tendencia se invierte en las regiones de la zona centro y sur del país, donde el porcentaje de halófitas introducidas supera el 50%. En los sistemas insulares este número es aún mayor, con valores sobre el 80%. El hábito predominante entre las halófitas chilenas es el herbáceo, con 114 especies que representan un 82,6% de la riqueza total de halófitas. Las hierbas perennes son el grupo mayoritario, con el 53% del total de las especies descritas (Tabla 2). Las especies halófitas leñosas constituyen un grupo menos abundante, con 5 árboles, 13 arbustos y 5 subarbustos, los que en conjunto representan casi el 17% del total de especies. De las 138 especies registradas sólo existe una especie con hábito de enredadera, Calystegia sepium (Anexo 1). Con respecto a la distribución regional de los distintos hábitos, las hierbas perennes son el hábito de mayor presencia en todas las regiones del país, con al menos un 40% de representación en cada una de ellas (Tabla 2). En la zona sur, este valor es aún mayor (60%).. 37.
(38) Las herbáceas anuales, corresponden al segundo grupo más abundante después de las herbáceas perennes en todas las regiones y presentan una mayor riqueza en regiones de la zona norte y centro, con una disminución progresiva hacia el sur (Tabla 2). La mayor frecuencia de especies leñosas (arbóreas, arbustivas y subarbustivas) ocurre en las regiones de Atacama y Coquimbo con 13 y 12 especies respectivamente (Tabla 2). En cambio, la frecuencia de este grupo de especies disminuye notoriamente hacia el extremo sur y en el territorio insular, con menos de 3 especies.. DISCUSIÓN Y CONCLUSIONES. RIQUEZA DE FAMILIAS HALÓFITAS Este es el primer estudio que compila la diversidad de especies halófitas presentes en Chile, a partir de un listado colaborativo mundial. En él se presentan 138 especies de halófitas, distribuidas en 31 familias, lo que equivale a un 2,3% de las especies y un 16,8% de las familias de flora vascular descritas para Chile (Marticorena 1990). Las familias más diversas son Poaceae (29 especies), Amaranthaceae (20 especies), Fabaceae (11 especies), Asteraceae (9 especies) y Cyperaceae (8 especies). Si bien esta representación coincide con algunas de las familias de mayor riqueza en Chile (Asteraceae, Poaceae y Fabaceae) (Comisión Nacional del Medio Ambiente, 2008), la familia Amaranthaceae no aparece en este. 38.
(39) grupo, a pesar de ser la segunda familia de mayor riqueza de halófitas a nivel nacional (Tabla 1) y la primera a nivel mundial (Santos et al. 2015). A nivel global, las 138 especies halófitas descritas en Chile representan un 9,3% de las 1479 especies identificadas por la base de datos eHALOPH (https://www.sussex.ac.uk/affiliates/halophytes/). Este valor está muy por debajo de las 673 especies nativas y endémicas descritas para Argentina a través de esta misma herramienta (Cantero et al., 2016) o las más de 300 especies descritas a partir de registros monográficos y trabajos de terreno en países de Europa (Grigore, 2008) y Asia (Khan & Qaiser 2006, Ghazanfar et al. 2014). La baja riqueza de halófitas a nivel nacional puede ser reflejo de la baja diversidad de especies en Chile comparada a la de otros países en Sudamérica (Comisión Nacional del Medio Ambiente, 2008); o bien, estar influenciada por la alta presencia de especies de origen euroasiático en los registros de la base de datos eHALOPH.. ORIGEN DE LAS ESPECIES Un 55% de las especies halófitas presentes en Chile son introducidas. Del 45% restante, un 42% corresponde a especies nativas, y sólo un 3% a especies endémicas (Tabla 2). Este patrón difiere significativamente al descrito para la flora vascular en Chile, compuesta por más de un 85% de especies nativas (Fuentes et al. 2013), de las que casi la mitad son endémicas (Moreira-Munoz, 2011). Al respecto, es importante considerar que la base de datos eHALOPH se construyó desde dos publicaciones (Aronson 1989, Menzel & Lieth 2003), cuyo objetivo fue la recopilación de especies tolerantes a la salinidad con potencial uso económico. 39.
(40) En ese sentido, no nos debe sorprender que, al contrastar este listado con la flora de Chile, se encuentre una presencia importante de especies introducidas, o que las familias con mayor diversidad de halófitas también posean la mayor riqueza de especies introducidas (Fuentes et al. 2013, Fuentes et al. 2014). Así también, la distribución multirregional de este grupo de especies (O’Leary & Glenn 1994) y el escaso conocimiento acerca de halófitas autóctonas en Chile (Ramírez & Álvarez 2012), puede ser otra causante de los resultados observados.. TABLA 2. Riqueza, hábito y origen de las especies halófitas presentes en Chile según su distribución geográfica. Distribución:. TAR= Región de Arica y. Parinacota, Región de Tarapacá; ANT= Región de Antofagasta; ATA= Región de Atacama; COQ= Región de Coquimbo; VAL= Región de Valparaíso; RME= Región Metropolitana; LBO= Región del Libertador General Bernardo O’Higgins; MAU= Región del Maule; BIO= Región del Biobío; ARA= Región de la Araucanía; LLA= Región de Los Ríos y Región de Los Lagos; AIS= Región de Aysén del General Carlos Ibáñez del Campo; MAG= Región de Magallanes y Antártica Chilena; IDE= Islas Desventuradas; IPA= Isla de Pascua; JFE= Archipiélago de Juan Fernández. Origen: EN= endémica; NA= nativa; IN= introducida. Forma de vida: HA= herbácea anual; HAB= herbácea anual o bianual; HP= herbácea perenne; Ar= arbustiva; Sa= subarbustiva; Ab= arbórea; E= enredadera perenne / Richness, habit and origin of halophyte species present in Chile by geographical distribution. Distribution: TAR= Arica y Parinacota Region, Tarapacá Region; ANT= Antofagasta Region; ATA= Atacama Region; COQ= Coquimbo Region; VAL= Valparaíso Region; RME= Metropolitan Region; LBO= Libertador Bernardo O’Higgins Region; MAU= Maule Region; BIO= Biobío Region; ARA= Araucanía Region; LLA= Los Ríos Region and Los Lagos Region; AIS= Aysén del General Carlos Ibáñez del Campo Region; MAG= Magallanes y Antártica chilena Region; IDE= Islas Desventuradas; IPA= Easter Island; JFE= Archipiélago de Juan Fernández. Origin: 40.
(41) EN= endemic; NA= native; IN= Introduced. Life form: HA= anual herb; HAB= anual or bianual herb; HP= perennial herb; Ar= shrub; Sa= subshrub; Ab= tree; E= perennial vine.. Región. Riqueza. Hábito (n° especies). Islas. Zona sur. Zona centro. Zona norte. HA. HP. Hab. Ab. Ar. Origen (n° especies) Sa. E. EN. NA. IN. TAR. 49. 11. 28. 1. 2. 4. 3. 0. 2. 25. 22. ANT. 58. 13. 32. 4. 2. 3. 4. 0. 2. 29. 27. ATA. 61. 15. 30. 3. 2. 8. 3. 0. 2. 30. 29. COQ. 81. 24. 42. 3. 2. 6. 4. 0. 2. 36. 43. VAL. 81. 23. 46. 4. 2. 3. 2. 1. 1. 32. 48. RME. 70. 19. 40. 4. 2. 3. 1. 1. 1. 29. 40. LBO. 44. 15. 18. 4. 2. 3. 2. 0. 0. 18. 26. MAU. 59. 18. 34. 3. 1. 2. 1. 0. 1. 23. 35. BIO. 74. 24. 39. 4. 1. 3. 2. 1. 0. 27. 47. ARA. 57. 17. 33. 3. 1. 1. 1. 1. 0. 20. 37. LLA. 58. 15. 36. 4. 0. 1. 1. 1. 0. 24. 34. AIS. 25. 5. 16. 2. 1. 0. 0. 1. 0. 12. 13. MAG. 37. 6. 25. 3. 0. 2. 1. 0. 1. 17. 19. IDE. 6. 2. 3. 0. 0. 1. 0. 0. 0. 0. 6. JFE. 26. 10. 12. 2. 1. 0. 1. 0. 0. 3. 23. IPA. 13. 2. 9. 0. 1. 0. 0. 1. 0. 2. 11. 138. 36. 73. 5. 5. 13. 5. 1. 4. 58. 76. 100. 26,1. 52,9. 3,6. 3,6. 9,4. 3,6. 0,7. 2,9. 42. 55,1. Total Chile % Total Chile. HÁBITO DE LAS ESPECIES HALÓFITAS PRESENTES EN CHILE Más del 80% de las especies halófitas identificadas en Chile son herbáceas. Este grupo, además, presenta la mayor riqueza en todas las regiones del país, incluso en el territorio insular (Tabla 2). Esta predominancia es llamativa, pues a nivel global, se ha descrito que la relación de riqueza entre halófitas leñosas y 41.
(42) herbáceas es más bien equitativa (Aronson 1989, Khan & Qaiser 2006, Ghazanfar et al. 2014). La alta representación de este grupo en Chile se puede explicar por la presencia de ciertos tipos de hábitat que favorecen la colonización de hierbas tolerantes a la salinidad, tales como ecosistemas costeros, humedales y lagunas salobres (San Martín et al. 1992, Teillier & Becerra 2003, Ramírez & Álvarez 2012). Al mismo tiempo, la alta proporción de especies introducidas en el grupo de hierbas halófitas puede ser un reflejo de su éxito en el uso de hábitat naturales y perturbados, tal como lo ha descrito Ramírez et al. (2018) en marismas de la Región del Biobío. Por otra parte, las halófitas leñosas en Chile apenas representan un 16,6% del listado total. Pero, al contrario de las herbáceas, la mayoría de estas (82,6%) son nativas o endémicas de las zonas norte y central (Tabla 2). La riqueza de especies leñosas en la zona norte se explica principalmente por la presencia de géneros nativos con dos o más especies (Atriplex, Prosopis, Lycium, Frankenia y Suaeda), cuya distribución regional se encuentra más bien restringida al territorio entre las regiones de Arica y Parinacota y Coquimbo (Anexo 1). En el norte, aparecen también tres de los cuatro árboles endémicos o nativos presentes en Chile: Prosopis tamarugo, P. chilensis y Geoffroea decorticans. Al respecto, se ha descrito que la condición de halofitismo entre especies arbóreas es rara (O’Leary & Glenn 1994). Aún más, si se considera que gran parte de los árboles halófitos descritos a nivel global crecen en manglares, y ecosistemas de interfaz marinoterrestre, sistemas radicalmente distintos a los que alojan a este grupo de especies en Chile.. 42.
(43) Al igual que las especies arbóreas, la diversidad de arbustos halófitos es bastante baja. Sin embargo, con una riqueza de 18 especies, 15 de las cuales son nativas y/o endémicas, vale la pena resaltar el componente autóctono de este grupo. Estas especies están representadas por variantes nativas y endémicas de géneros con amplia distribución mundial, como Lycium, Atriplex, Suaeda y Senecio. Esto podría indicar que, dentro de la gran diversidad de estos géneros, ocurre cierto nivel de adaptación local a ecosistemas salinos.. PERSPECTIVAS PARA EL ESTUDIO DE HALÓFITAS EN CHILE Hoy en día, existe amplio conocimiento acerca de la fisiología y las estrategias de adaptación que utilizan las especies halófitas para enfrentarse a distintos sistemas salinos (Munns & Tester 2008). Sin embargo, existe un gran vacío de información con respecto a su diversidad a nivel nacional y a las distintas estrategias que utilizan para adaptarse a este tipo de ambientes en Chile. Al realizar una búsqueda online de libros y artículos científicos con las palabras “halófita” y “Chile”, se encuentran listados de especies que existen en hábitats salinos, análisis paleobotánicos de ecosistemas antiguos, estudios de acumulación y tolerancia a metaloides y estudios acerca del uso de halófitas para la nutrición humana y animal. La mayoría de estos artículos fueron publicados entre 1980 y 2000 y denotan la falta de información sistematizada sobre la riqueza de especies halófitas presentes en Chile y de sus características ecológicas. En este contexto, el presente listado, realizado a partir de los registros obtenidos en la. 43.
(44) base de datos eHALOPH, se constituye como una primera aproximación para compilar la diversidad de las especies halófitas presentes en Chile. Sin embargo, el enriquecimiento de este listado, ya sea a través de una exhaustiva revisión de fuentes bibliográficas nacionales, y la generación de nuevos aportes a dicha base de datos, requiere de la activa colaboración de investigadores y académicos. De este modo, identificar nuevas especies halófitas y ampliar nuestro conocimiento ecológico acerca de ellas, nos permitirá también identificar nuevas oportunidades para relevar la importancia de esta parte del patrimonio natural a través de su uso en diferentes áreas.. AGRADECIMIENTOS. Proyecto CONICYT FB-0002-2014 (Center of Applied Ecology and Sustainability, CAPES UC). Beca CONICYT doctorado 21141059. REFERENCIAS AL-FARRAJII, F., AL-HILLI, M.R. 1994. Halophytes and desertification control in Iraq. In: Squires V.R., Ayoub A.T (eds.), Halophytes as a resource for livestock and for rehabilitation of degraded lands, pp. 239-248. Springer, Switzerland. ARONSON, J.A. 1989. Salt Tolerant Plants of the World. Office of Arid Land Studies, University of Arizona. Arizona, U.S.A. 77 pp. ARONSON, J., LE FLOC’H, E. 1995. Restoration ecology of salt-affected, arid and semi-arid lands. En: Choukr-AllAh (ed.), Halophytes and biosaline agriculture, pp. 55-71; 53. CRC Press, U.S.A.. 44.
(45) BARTELS, D., DINAKAR, C. 2013. Balancing salinity stress responses in halophytes and non-halophytes: A comparison between Thellungiella and Arabidopsis thaliana. Functional Plant Biology 40:819-831. BRECKLE, S. 1990. Salinity tolerance of different halophyte types. In: El Bassam, N., Dambroth, M., Loughman, B.C. (eds.), Genetic Aspects of Plant Mineral Nutrition. Developments in Plant and Soil Sciences, Vol. 42, pp. 167-175. Springer, Dordrecht, Netherlands. BUHMANN, A.K., WALLER, U., WECKER, B., PAPENBROCK, J. 2015. Optimization of culturing conditions and selection of species for the use of halophytes as biofilter for nutrient-rich saline water. Agricultural Water Management 149: 102-114. CANTERO, J.J., PALCHETTI, V., NÚÑEZ, C., BARBOZA, G. 2016. Halophytic flora of Argentina: a checklist and an analysis of its diversity. In: Khan, M.A., Boër, B., Ȫzturk, M., Clüsener-Godt, M., Gul, B., Breckle, S.-W. (eds.), Sabkha ecosystems, pp. 137-204. Springer, Switzerland. COMISIÓN NACIONAL DEL MEDIO AMBIENTE. 2008. Biodiversidad de Chile, Patrimonio y Desafíos. Editorial Ocho Libros, Santiago, Chile. 640 pp. FLOWERS, T.J., COLMER, T.D. 2008. Salinity tolerance in halophytes. New Phytologist 179(4): 945-963. FLOWERS, T.J., HAJIBAGHERI, M., CLOPSON, N.J.W. 1986. Halophytes. The Quarterly Review of Biology 61(3): 313-337. FUENTES, N., PAUCHARD, A., SÁNCHEZ, P., ESQUIVEL, J., MARTICORENA, A. 2013. A new comprehensive database of alien plant species in Chile based on herbarium records. Biological Invasions 15(4): 847-858. FUENTES, N., SÁNCHEZ, P., PAUCHARD, A., URRUTIA, J., CAVIERES, L., MARTICORENA, A. 2014. Plantas invasoras del centro-sur de Chile: una guía de campo. Laboratorio de Invasiones Biológicas (LIB), Concepción, Chile. 276 pp. GHAZANFAR, S.A., ALTUNDAG, E., YAPRAK, A.E., OSBORNE, J., TUG, G.N., VURAL, M. 2014. Halophytes of Southwest Asia. En: Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B. (eds), Sabkha Ecosystems, Vol 4: Cash Crop Halophyte and Biodiversity Conservation, pp. 105-133. Springer, Dordrecht, The Netherlands. GRIGORE, M. 2008. Halophytotaxonomy: list of romanian salt tolerant plants. PIM, Iasi. 137 pp. GRIGORE, M., IVANESCU, L., TOMA, C. 2014. Halophytes and their habitats: finding a place within plant ecological classes. En: Grigore, M., Ivanescu, L., Toma, C., Halophytes: an integrative anatomical study, pp. 27-31. Springer, Cham. GRIGORE, M., TOMA, C., BOSCAIU, M. 2010. Dealing with halophytes: an old problem, the same continuous exciting challenge. Analele Stiintifice ale Universitatii “Al.I. Cuza” din Iasi 56(1): 21-32. 45.
(46) KHAN, M., QAISER, M. 2006. Halophytes of Pakistan: characteristics, distribution and potential economic usages. In: Khan, M.A., Böer, B., Kust, G.S., Barth, H.J. (eds.), Sabkha Ecosystems, Vol 2: West and Central Asia, pp. 129-153. Springer, Dordrecht, The Netherlands. KOYRO, H.W. 2003. Study of potential cash crop halophytes by a quick check system: Determination of the threshold of salinity tolerance and the ecophysiological demands. En: Cash crop halophytes: recent studies, pp. 5-17. Springer, Dordrecht. LAILHACAR, S., HUGO, R., SILVA, H., CALDENTEY, J. 1995. Rendimiento de leña y recuperación al corte en diferentes especies y procedencias arbustivas del género Atriplex. Revista de Ciencias Forestales (Chile) 10: 85-97. LUTTS, S., LEFEVRE, I. 2015. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Annals of Botany 115: 509-528. MARTICORENA, C., QUEZADA, M. 1985. Catálogo de la flora vascular de Chile. Gayana Botánica 42 (1-2): 1-157. MARTICORENA, C. 1990. Contribución a la estadística de la flora vascular de Chile. Gayana Botánica 47 (3-4): 85-113. MATTHEI, O., MARTICORENA, C., RODRÍGUEZ, R. 1995. Manual de las Malezas que Crecen en Chile. Editorial Alfabeta, Chile. 545 pp. MENZEL, U., LIETH, H. 2003. Halophyte Database V 2.0 update. In: Lieth H., Mochtchenko, M. (eds.), Cash Crop Halophytes: Recent Studies: 10 Years after Al Ain Meeting, Vol. 38 pp. 231. Springer, U.S.A MINISTERIO DE MEDIO AMBIENTE. 2017. Inventario Nacional de Especies de Chile. URL: http://especies.mma.gob.cl/CNMWeb/Web/WebCiudadana/Default.aspx. Accedido: 16 de diciembre 2017. MOREIRA-MUNOZ, A. 2011. Plant Geography of Chile. Springer Science & Bussiness Media, New York, U.S.A. 343 pp. MUNNS, R., TESTER, M. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59 (1): 651-681. O’LEARY, J., GLENN, E.P. 1994. Global distribution and potential for halophytes. In: Squires, V., Ayoub, A.T. (eds.), Halophytes as a resource for livestock and for rehabilitation of degraded lands. pp 7-18. Springer, Dordrecht. POBLETE, V., CAMPOS, V., GONZALEZ, L. 1991. Anatomical leaf adaptations in vascular plants of a salt marsh in the Atacama Desert (Chile). Revista Chilena de Historia Natural 64: 65-75. RAMÍREZ, C., ÁLVAREZ, M. 2012. Flora y vegetación hidrófila de los Humedales Costeros de Chile. En: Fariña, J.M., Camaño, A. (eds.), Humedales costeros de 46.
(47) Chile: Aportes científicos a su gestión sustentable, pp. 101-145. Ediciones UC, Santiago, Chile. RAMÍREZ, C., SAN MARTÍN, C. 2006. Diversidad de macrófitos chilenos. En: Vila, I., Veloso, A., Schlatter, R., Ramírez, C. (eds.), Macrófitas e invertebrados de los sistemas líminicos de Chile. pp. 21-61. Editorial Universitaria, Santiago, Chile. RAMÍREZ, C., FARIÑA, J.M., CAMAÑO, A., SAN MARTÍN, C., PÉREZ, Y., SOLÍS J.L., VALDIVIA, O. 2018. The case of the Itata estuary (Bio-Bio Region-Chile) plant formations: anthropogenic interference or natural disturbance-induced diversity enrichment? Mediterranean Botany 39(1): 17-34. RHODES, D., FELKER, P. 1988. Mass screening of Prosopis (mesquite) seedlings for growth at seawater salinity concentrations. Forest Ecology and Management 24(3): 169-176. SAN MARTÍN, C., CONTRERAS, D., SAN MARTIN, J., RAMIREZ, C. 1992. Vegetación de las marismas del centro-sur de Chile. Revista Chilena de Historia Natural 65: 327-342. SANTOS, J., MOHAMMED, A.A., ARONSON, J., FLOWERS, T.J. 2015. eHALOPH a database of salt-tolerant plants: helping put halophytes to work. Plant and Cell Physiology 57(1): e10. SHPIGEL, M., BEN-EXRA, D., SHAULI, L., SAGI, M., VENTURA, Y., SAMOCHA, T., LEE, J.J. 2013. Constructed wetland with Salicorina as a biofilter for mariculture effluents. Aquaculture 412: 52-63. TEILLIER, S. 1998. Flora y vegetacion alto andina del area de collaguasi salar de coposa andes del norte de chile. Revista Chilena de Historia Natural 71: 313-329. TEILLIER, S., BECERRA, P. 2003. Flora y vegetacion del salar de Ascotan, andes del norte de Chile. Gayana Botánica 60: 114-122. TODERICH, K.N., ISMAIL, S., JUYLOVA, E.A., RABBIMOV, A.A., BEKCHANOV, B.B., SHYUSKAYA, E.V., GISMATULLINA, L.G., OSAMU, K., RADJABOV, TB. 2008. New approaches for biosaline agriculture development, management and conservation of sandy desert ecosystems. In: Abdelly, Ch., Öztürk, M., Ashraf, M., Grignon C. (eds.), Biosaline Agriculture and High Salinity Tolerance, pp. 247-264. Springer, Switzerland. ZULOAGA, FO., MORRONE, O., BELGRANO, M.J. 2008. Catálogo de las plantas vasculares del Cono Sur: (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay). Missouri Botanical Garden Press, St. Louis, U.S.A. 3486 pp.. 47.
Figure



Documento similar
It was found that shredding (wounding stress) and hyperoxia storage (80 kPa O 2 ) induced the highest phenylalanine ammonia-lyase (PAL), TPC and TAC enhancements in carrots
halimus remains a vital plant species in low-rainfall regions (Walker et al., 2014). halimus should not be considered for phytostabilization, and has the potential to be
gondii infection in insectivorous bats [15] and multiple risk factors, other than trophic sources, have been proposed: different ecological traits of bat species
To use response statements in these ways is to make them more than records of subjective reactions; it is to devise ways of integrating more traditional
Predation was corrected for control mortality using the formula proposed by Xia et al. Functional response of each predator species was then analyzed in two steps: i)
We considered both biotic–biotic and abiotic–biotic correlations, and used a total of 14 abiotic and biotic ecosystem constituents: soil bulk density, pH, carbon (C) content,
In medicinal products containing more than one manufactured item (e.g., contraceptive having different strengths and fixed dose combination as part of the same medicinal
The peak response was just after the hand had passed the second target in 50% of the trials (vertical dashed lines) and was larger than the response to the first object jumping,
