DOI: 10.1080/08830180802276179
Special Topic: NF-
κ
B, Immunity and Cancer
NF-
κ
B Signaling Pathway and Its Therapeutic
Implications in Human Diseases
Fazlul H. Sarkar, Yiwei Li, Zhiwei Wang, and Dejuan Kong
Department of Pathology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, Michigan, USA
The nuclear factor-κB (NF-κB) pathway is one of the most important cellular sig-nal transduction pathways involved in both physiologic processes and disease conditions. It plays important roles in the control of immune function, inflam-mation, stress response, differentiation, apoptosis, and cell survival. Moreover, NF-κB is critically involved in the processes of development and progression of cancers. More importantly, recent studies have shown that NF-κB signaling also plays critical roles in the epithelial-mesenchymal transition (EMT) and cancer stem cells. Therefore, targeting of NF-κB signaling pathway could be a potent strategy for the prevention and/or treatment of human cancers and inflammatory diseases.
Keywords NF-κB, immune, inflammation, cancer, EMT
NUCLEAR FACTOR-
κ
B (NF-
κ
B) PATHWAY
NF-κB was firstly discovered as a factor bound to theκlight-chain im-munoglobulin enhancer in the nuclei of B lymphoid lineage [1]. Shortly after, NF-κB was found in PMA-treated Jurkat and HeLa cells, sug-gesting that NF-κB is non–cell-type-specific and inducible [2]. With the discovery of its activators and downstream targets, NF-κB pathway has become one of the most intensely studied signaling pathways in the past three decades. Emerging evidence demonstrates that NF-κB signaling pathway plays important roles in the control of cell growth, differentiation, apoptosis, immune, inflammation, stress response, and
Address correspondence to Fazlul H. Sarkar, Department of Pathology, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, 740 Hud-son Webber Cancer Research Center, 4100 John R, Detroit, MI 48201, USA. E-mail: fsarkar@med.wayne.edu
293
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
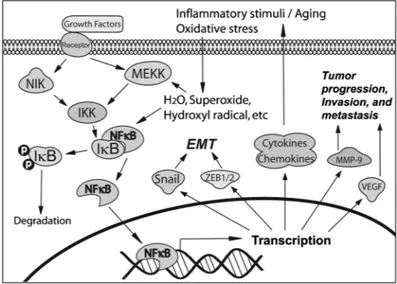
many other physiologic processes [3, 4]. The NF-κB family has five pro-teins: RelA (p65), RelB, Rel, NF-κB1 (p50), and NF-κB2 (p52), each of which may form homo- or heterodimers. The activated NF-κB is a het-erodimer, which usually consists of p65 and p50 or contains p52 and RelB. In human cells without specific extracellular signal, NF-κB is se-questered in the cytoplasm through tight association with its inhibitors: IκBα, which acts as a NF-κB inhibitor, and p100 proteins, which serve as both inhibitor and precursor of NF-κB DNA-binding subunits. NF-κB can be activated by many types of stimuli such as cytokines, oxidants, viruses, immune stimuli, and so forth [4]. The activation of NF-κB oc-curs through phosphorylation of IκBαby IKKβand/or phosphorylation of p100 by IKKα, leading to the degradation of IκBαand/or the process-ing of p100 into smaller form (p52). This process allows two forms of activated NF-κB (p50-p65 and p52-RelB) to become free, resulting in the translocation of the activated NF-κB into the nucleus for binding to NF-κB–specific DNA-binding sites, which, in turn, regulates the tran-scription of target genes (Fig. 1) [3]. In addition, IKKαalso regulates the activation of NF-κB–directed gene expression through phosphorylation of histone H3 [5].
By binding to the promoters of target genes, NF-κB controls the expression of many genes that are involved in the processes of immu-nity, inflammation, cell survival, and apoptosis. The activated NF-κB frequently functions together with other transcription factors such as AP-1 to regulate the expression of immune and inflammatory genes[6,7]. NF-κB has been found to promote the expression of cy-tokines, chemokines, and receptors including IL-2, IL-6, IL-8, TNF-α, IL-2R, TCR, and so forth [4, 6, 7]. These proteins regulated by NF-κB also cause the activation of NF-κB, forming a positive regulatory loop to amplify inflammatory responses [4]. NF-κB also transcriptionally upregulates 5-lipoxygenase, cyclooxygenase-2 (COX-2), and nitric ox-ide synthase (NOS) [8–10], which are inflammatory enzymes and play important roles in the processes of inflammation. It has been reported that overexpression of NF-κB protects cells from apoptosis whereas inhibition or absence of NF-κB induces apoptosis or sensitizes cells to apoptosis-inducing agents including TNF-α, ionizing radiation can-cer therapeutic agents, and so forth [11, 12]. NF-κB can also bind to the promoters and enhancers of some target genes (i.e., MMP-9, IL-8, uPA, VEGF, CXCR4, osteopontin, etc.) that control cancer cell in-vasion, metastasis, and angiogenesis [13–15]. These findings suggest that NF-κB is a critical transcription factor for the control of immu-nity, inflammation, cell survival, apoptosis, and cancer cell invasion and metastasis (Fig. 1).
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
FIGURE 1 NF-κB pathway and its role in human diseases.
OXIDATIVE STRESS AND NF-
κ
B ACTIVATION
It is well-known that NF-κB is critically involved in the processes of oxidative stress response. Oxidative stress is defined as an increase in intracellular reactive oxygen species (ROS) such as H2O2, superoxide,
hydroxyl radical, and so forth. We and other investigators have found that direct addition of H2O2to cell culture medium activates NF-κB in
several types of cell lines [16, 17]. In addition, ROS in cells are increased in response to agents that also activate NF-κB [18]. These results sug-gest that oxidative stress activates NF-κB activity in the cells.
Oxidative stress is frequently observed as part of aging, immune dis-orders, inflammatory diseases, metabolic diseases, and cancers. When ROS are intracellularly formed, the activity of NF-κB is upregulated, leading to the expression of cytokines, chemokines, cellular adhesion molecules, and inflammatory enzymes that activate the immune and inflammatory system. Therefore, the inflammatory reactions and al-tered immune responses occurring in inflammatory diseases are often associated with an increase in ROS production, activation of NF-κB, and expression of NF-κB driven proteins. In addition to the induc-tion of inflammatory factors, ROS also induce DNA damage and alter cellular signal transduction pathways among which NF-κB signaling is the most important. Because DNA damage plays a central role in carcinogenesis, it is conceivable that oxidative stress could be carcino-genic [19]. Hence, DNA repair is an important process in preventing
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
mutagenesis. However, under oxidative stress, the repair of DNA dam-age can be inhibited by several redox-dependent metals, resulting in carcinogenesis [19]. Moreover, the activation of NF-κB has been known as a key event in carcinogenesis [20], suggesting the importance of NF-κB activation in the development of cancer.
ACTIVATION OF NF-
κ
B IN AGING AND METABOLIC DISEASES
Because human beings live in an aerobic environment, exposure to ROS is continuous and unavoidable with aging. Although a number of defense systems have evolved to combat the accumulation of ROS, these defense systems are not always adequate to counteract the pro-ductions of ROS, resulting in oxidative stress. Oxidative stress and NF-κB activation have been linked to the normal aging and a variety of chronic metabolic diseases including diabetes, pulmonary fibrosis, neurodegenerative diseases, and so forth [21].
There is growing evidence implicating NF-κB activation and oxida-tive damage to DNA, protein, and lipid in the pathogenesis of vari-ous chronic diseases. Type I diabetes, or insulin-dependent diabetes, is characterized by the severe destruction of insulin-producingβcells, and ROS are believed to play a central role inβ-cell death and disease progression [22]. In diabetes, pancreas-specific ROS production plays a critical role in signal transduction response by activating NF-κB. Both c-Rel and NF-κB1 are essential for the development of type I diabetes [23,24]. Alzheimer’s disease is the most common neurodegenerative disease and shows progressive memory loss and dementia. Growing evidence shows that there is a linkage between Alzheimer’s disease and oxidative damage with activated NF-κB [25, 26]. Administration of vitamin E leads to a slowing of disease progression, and the patients taking antioxidant vitamins and anti-inflammatory compounds have a lower incidence of Alzheimer’s disease [25], suggesting the importance of NF-κB activity in the disease process. Parkinson’s disease is the sec-ond most common neurodegenerative disease and causes a progressive movement disorder. The oxidative damage to protein, the protein oxida-tion, and the activated NF-κB have been found in Parkinson’s disease, suggesting the involvement of NF-κB signaling pathway in neurodegen-erative disease [25, 27], and active research is ongoing in these areas.
ACTIVATION OF NF-
κ
B IN INFLAMMATORY DISEASES
NF-κB has been involved in the differentiation of macrophages, osteo-clasts, granulocytes, and T and B cells, all of which play important roles
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
in immunity and inflammation. During the development of the adaptive immune system, NF-κB signaling is constitutively activated via an au-tocrine loop induced by chemokines, cytokines and their respective re-ceptors, leading to the differentiation of macrophages, granulocytes, T and B cells, as well as for the maintenance of their homeostasis. Be-cause these cells are critically involved in the processes of inflamma-tion, NF-κB is known as a primary regulator of inflammatory response. The activation of NF-κB has been found in human inflammatory dis-eases such as rheumatoid arthritis [28], atherosclerosis [29], gastritis [30], inflammatory bowel disease [31], sepsis [32], systemic lupus ery-thematosus [33], and so forth. It has been reported that ROS contribute significantly to tissue injury in rheumatoid arthritis, and that the con-trol of inflammation in arthritic patients by antioxidants could become a relevant strategy for antirheumatic therapy [34]. The increase in ROS generation has also been related to the risk for cardiovascular diseases such as atherosclerosis, angina pectoris, and myocardial infarction [35], suggesting the activation of NF-κB in these diseases. The activity of NF-κB and the expression of proinflammatory cytokines are increased in most of the inflammatory diseases and further causes deregulation of the immune response [30–32].
Inhibition of NF-κB activation is now widely recognized as a valid drug-target strategy to combat inflammatory disease [36]. However, it has become obvious that the inhibition of NF-κB activity is not only desirable for the treatment of inflammation but also in cancer therapy [37]. It is hypothesized that chronic inflammation generates a microenvironment that contributes to malignant transformation in hu-man organs [38]. Examination of the inflammatory microenvironment in neoplastic tissues has supported the hypothesis that inflammation is a cofactor in oncogenesis for a variety of cancers. Moreover, the inflam-matory mediators such as cytokines, prostaglandins, and growth fac-tors, which are regulated by NF-κB, can induce genetic and epigenetic changes including mutations in tumor suppressor genes, DNA methyla-tion, and post-translational modifications, leading to the development and progression of cancer [39]. Many anti-inflammatory drugs and an-tioxidants can inhibit NF-κB activity and induce apoptosis; therefore, they may also be desirable for the treatment of cancers.
ACTIVATION OF NF-
κ
B IN CANCERS
NF-κB has been described as a major culprit in cancer because it is con-stitutively activated in most human cancers, especially in the poorly differentiated cancers, but not in normal tissues and immortalized cells
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
[20]. It has been reported that NF-κB is activated in Hodgkin’s tumor [40] and multiple myeloma cells [41]. The activation of NF-κB is also frequently observed in breast and prostate cancer cells [42, 43]. Stud-ies have also shown that NF-κB is upregulated in pancreatic cancer [44, 45], gastric carcinoma [46], and squamous cell carcinoma of the head and neck [47]. In cancer cells, NF-κB is activated through constitu-tive activation of IKK followed by the phosphorylation and degradation of IκBα. Inhibition of NF-κB by a super-inhibitor of NF-κB (δ-N-IκBα) results in impaired proliferation and induction of apoptosis [48], sug-gesting that NF-κB is a target for the treatment of cancer.
Moreover, evidence has shown that NF-κB participates in the pro-cesses of cancer metastasis. It was found that inhibition of constitutive NF-κB activity by a mutant IκBα (S32A, S36A) completely sup-pressed the liver metastasis of pancreatic cancer cells [49]. The in-hibition of NF-κB activity also inhibited the tumorigenic phenotype of a nonmetastatic pancreatic cancer cell, suggesting that constitu-tive NF-κB activity plays a key role in cancer metastasis and tumor progression [50]. Constitutive NF-κB activation also increases the ex-pression of its downstream genes, uPA and MMP-9, both of which are the critical proteases involved in tumor invasion and metasta-sis [51]. These results suggest that constitutively activated NF-κB is tightly related to the invasion and metastatic processes during tumor progression.
De novo and acquired resistance to chemotherapeutic agents is a major cause of treatment failure in cancer. It has been found that chemotherapeutic agents can activate NF-κB in cancer cells, leading to cancer cell resistance to chemotherapy [52–54]. Other molecules such as IL-1 and E3-ubiquitin ligase receptor could also induce NF-κB activation and lead to the chemoresistance in human cancer cells, suggesting that NF-κB plays important roles in the development of chemoresistance in cancers [55,56]. Therefore, it is becoming obvious that inhibition of NF-κB activity is highly desirable for the treatment of chemoresistant cancer.
NF-
κ
B ACTIVATION AND EPITHELIAL-MESENCHYMAL
TRANSITION (EMT)
EMT was recognized as a critical feature of embryogenesis several decades ago; however, more recently it has also been implicated in cancer progression. During EMT of cancer cells, expression of pro-teins that promote cell-cell contact such as E-cadherin and γ-catenin is lost, and there is gain in the expression of mesenchymal markers
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
such as vimentin, fibronectin, N-cadherin, MMP-2, and MMP-9, which leads to enhanced ability of cancer cell migration and invasion [57]. The epithelial cancer cells undergo remarkable morphologic changes characterized by a transition from epithelial cobblestone phenotype to elongated fibroblastic phenotype with the loss of epithelial cell-cell junc-tion and actin cytoskeleton reorganizajunc-tion. For most epithelial tumors, progression toward malignancy is accompanied by a loss of epithelial differentiation and a shift toward mesenchymal phenotype [57]. More-over, studies have shown that primary cancers and their corresponding metastatic tumors exhibited mixed epithelial-mesenchymal phenotype [58]. Cells in the tumor center remain positive for the expression of E-cadherin and cytoplasmicβ-catenin, and the tumor cells in the periph-ery display loss of surface E-cadherin and upregulation of vimentin, the typical characteristics of EMT phenotype [58]. The alteration of molecules during EMT has been observed in association with dysreg-ulated cellular signal transduction pathways. The downregulation and relocation of E-cadherin and zonula occludens-1 (ZO-1), the transloca-tion ofβ-catenin from cell membrane to nucleus, and the activation of Snail, Twist, and ZEB1 transcription factors results in the induction of EMT phenotype [59–64].
Importantly, increasing evidence has shown that NF-κB activation is required for the induction and maintenance of EMT (Fig. 1) [65,66]. It has been found that NF-κB suppresses the expression of epithelial spe-cific genes, E-cadherin and desmoplakin, and induces the expression of the mesenchymal specific gene vimentin [67]. Repression of E-cadherin expression by the transcription factor Snail is a central event during the loss of epithelial phenotype. NF-κB has been found to induce the expression of Snail, leading to the downregulation of E-cadherin [68]. NF-κB also upregulates transcription factor ZEB1 and ZEB2, resulting in the inhibition of E-cadherin expression during EMT [67]. Further-more, studies have shown that NF-κB could induce Bcl-2 expression and promote invasion of breast cancer cells, which are associated with EMT [69]. Recently, we have found that overexpression of PDGF-D could activate NF-κB and thereby upregulates Bcl-2, which may con-tribute to EMT phenotype and invasive behavior of PC-3 prostate can-cer cells [70]. In addition, gene expression profile analysis has shown that the genes involved in EMT and NF-κB signaling deregulation are the most prominent molecular characteristics of the high-risk head and neck squamous cell carcinoma [71], suggesting that NF-κB signaling plays important roles in EMT. Therefore, inhibition of NF-κB could stop the progression, invasion, and metastasis of cancer in part due to deregulation of the processes of EMT.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
TARGETING NF-
κ
B FOR THE TREATMENT OF HUMAN
DISEASES
Because NF-κB is constitutively activated in many human cancers, immune disorders, inflammation, and metabolic diseases, targeting NF-κB signaling is an important strategy for the treatment of many human diseases. Experimental studies have shown that natural an-tioxidant compounds including isoflavones, curcumin, green tea cate-chins, and so forth, inhibit the activity of NF-κB and induce apoptotic cell death in cancers and inflammatory diseases. Moreover, synthetic NF-κB inhibitors also suppress inflammatory diseases and tumor growth through inhibition of NF-κB activity. Thus, natural or synthetic antioxidants that inactivate NF-κB activity could serve as molecularly targeted agents against human diseases.
Natural Antioxidant Compounds
Isoflavones
Isoflavones are a subclass of flavonoids. Genistein, daidzein, and glycitein are three main isoflavones found in soybeans. Evidence from epidemiologic andin vivostudies has shown a decreased risk of cancer with soy consumption [72,73]. Experimental studies have revealed that isoflavones, particularly genistein, exert antioxidant and anti-NF-κB effects on human inflammatory and cancer cells.
In lipopolysaccharide-induced inflammation, isoflavones including genistein, kaempferol, quercetin, and daidzein have been found to in-hibit the activation of NF-κB with downregulation of iNOS expression and NO production in activated macrophages [74], suggesting their anti-inflammatory effect. Isoflavone genistein also prevents NF-κB activation and acute lung injury induced by lipopolysaccharide in Sprague-Dawley rats [75]. Dietary supplementation with soy isoflavone also reduces the exhaled NO level, the inflammatory mediators in the exhaled breath condensate, and the ability of eosinophils to release in-flammatory molecules in asthma [76, 77]. These results provide some evidence demonstrating the inhibitory effects of isoflavones on inflam-mation.
We have investigated the effects of genistein on NF-κB DNA binding activity in prostate, breast, and pancreatic cancer cells. We found that genistein treatment significantly inhibited NF-κB DNA-binding activ-ity in all cell lines we tested [53, 78, 79]. We also found that genistein significantly inhibited cancer cell growth and induced apoptotic cell death through the downregulation of NF-κB [53, 78, 79]. More impor-tantly, we and other investigators have found that isoflavone could
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
enhance the therapeutic effects of chemotherapy and radiotherapy through the inhibition of NF-κB [53,80]. These results suggest that isoflavone functions as an antioxidant, which could be a potent agent for the inhibition of cancer cell growth.
By in vivo studies, we found that isoflavone supplementation in-hibited NF-κB DNA binding activity and abrogated TNF-α–induced NF-κB activity in lymphocytes harvested from human subjects receiv-ing soy isoflavone supplements [17]. We also found that isoflavone is very effective in reducing the level of 5-OhmdU, a modified DNA base that represents the endogenous status of cellular oxidative stress [17]. These results demonstrated that isoflavone inhibits NF-κB activation and decreases oxidative damage, suggesting its inhibitory effects on carcinogenesis. Several phase I and II clinical trials using isoflavone as supplement in the treatment of prostate, breast, and bladder cancers have also been or are being conducted [81] (www.clinicaltrials.gov). The results of clinical trials will hopefully guide us to design novel strategy for the treatment of cancer.
Curcumin
Curcumin is a compound fromCurcuma longa(tumeric).It has re-cently received considerable attention due to its pronounced anti-inflammatory, antioxidative, immunomodulating, antiatherogenic, and anticarcinogenic activities [82,83]. It has been found that curcumin inhibits IKK, suppresses both constitutive and inducible NF-κB acti-vation, and potentiates TNF-α–induced apoptosis [84]. Curcumin also showed strong antioxidant and anticancer properties through regulat-ing the expression of genes that require the activation of NF-κB [85], suggesting that curcumin is a strong inhibitor of NF-κB.
Curcumin has been shown to inhibit diethylnitrosamine-induced liver inflammation and trinitrobenzene sulfonic acid–induced colitis through the inhibition of NF-κB in rats and mice [86, 87]. Curcumin also inhibits NF-κB activation and reduces the severity of experimental steatohepatitis in mice [88]. It has been found that curcumin attenu-ates inflammatory responses of TNF-α–stimulated human endothelial cells [89] and protects against acute liver damage by inhibiting NF-κB, proinflammatory cytokines production, and oxidative stress [90]. Obe-sity is a major risk factor for the development of type 2 diabetes. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabetes [91]. These findings suggest the inhibitory effects of curcumin on inflammatory and metabolic diseases. Curcumin also showed strong anticancer properties mediated through the regulation of the expression of genes that require the
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
activation NF-κB [92]. We have also found that curcumin inhibited cell growth and induced apoptosis in pancreatic cancer cells in part due to downregulation of Notch-1, Hes-1, and NF-κB [93]. Moreover, curcumin downregulates the inflammatory cytokines CXCL-1 and -2 in breast cancer cells via NF-κB [94]. Curcumin also inhibits tumor growth in ovarian carcinoma, head and neck squamous cell carcinoma, and colon cancer by targeting the NF-κB pathway [95–97]. Importantly, curcumin has been found to significantly inhibit chemotherapeutic agent–induced NF-κB activation and enhance the antitumor activities of chemotherapeutics through inhibition of NF-κB in different types of cancers [52,98]. These results suggest that curcumin could inhibit can-cer cell growth and sensitize cancan-cer cells to chemotherapeutic agents through the inhibition of the NF-κB pathway.
Two phase I trials using curcumin have been conducted to test the safety, biomarkers, and activity of curcumin. The results demonstrated that PGE2production in blood and target tissue could indicate the
bi-ological activity of curcumin [99] and that curcumin showed a biologic effect on the chemoprevention of cancer [100]. Recently, oral curcumin has been used for a phase II clinical trial in patients with pancreatic cancer [101]. The authors have found that curcumin has potent NF-κB inhibitory effects, and it has shown activity to inhibit NF-κB in pe-ripheral blood mononuclear cells (PBMCs). The authors also showed that 2 of 49 patients had benefit and another patient had more than 2 years of stable disease [101]. Because oral curcumin showed some activity, further studies have been done using liposome-encapsulated formulation of curcumin. This formulation showed significant antitu-mor, antiangiogenesis, and NF-κB inhibitory effects in pancreatic can-cer in mouse xenograft model [102,103]. In addition, a phase III clinical trial using combination of gemcitabine, curcumin, and celebrex in pa-tients with advance or inoperable pancreatic cancer is being conducted. This trial will be able to demonstrate whether the combination effect could be correlated with synergistic augmentation of apoptosis involv-ing downregulation of COX-2 protein. Another clinical trial is beinvolv-ing conducted to determine whether supplementation of curcumin could decrease oral mucositis in children undergoing doxorubicin-containing chemotherapy (www.clinicaltrials.gov). In addition, a pilot study of cur-cumin and ginkgo for treating Alzheimer’s disease is being conducted (www.clinicaltrials.gov).
Indole-3-carbinol and 3,3’-Diindolylmethane
Indole-3-carbinol (I3C) is produced from naturally occurring glucosi-nolates contained in a wide variety of plants including members of the
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
family Brassica. I3C is biologically active and is easily convertedin vivo
to its dimeric product 3,3-diindolylmethane (DIM). I3C and DIM have been shown to reduce oxidative stress and stimulate antioxidant re-sponse element–driven gene expression as antioxidants [104, 105].
DIM has been found to suppress the inflammatory response to lipopolysaccharide (LPS) in murine macrophages. DIM suppresses LPS-induced NF-κB transcriptional activity by inhibiting degradation of IκBα, translocation of NF-κB to the nucleus, and NF-κB DNA-binding activity [106]. DIM also significantly decreases the release of NO, PGE2, COX-2, TNF-α, IL-6, and IL-1βby RAW264.7 cells treated with LPS [106]. Downregulation of NF-κB could be one of the mechanisms by which DIM inhibits inflammatory responses. NO, PGE2, and COX-2 play pivotal roles as mediators of inflammation involved in early steps of carcinogenesis. Therefore, the inhibition of these molecules by DIM, through inhibition of NF-κB, could also suppress the onset of cancer. Indeed, rats fed with I3C, precursor of DIM, show decreasing tendency in multiplicities of colonic adenomatous polyps [107].
We have found that both I3C and DIM significantly inhibited NF-κB DNA binding activity in cancer cells, corresponding with the inhibition of cell proliferation and the induction of apoptosis [108, 109]. Importantly, it has been found that the combinations of I3C and cisplatin or tamoxifen cooperate to inhibit the growth of PC-3 prostate and MCF-7 breast cancer cells more effectively than does either agent alone [110, 111], suggesting that inhibition of NF-κB activity by I3C and DIM may contribute to the enhanced antitumor activity of chemotherapeutic agents.
Several clinical trials using I3C and DIM in the treatment of various cancers have been or are being conducted [112, 113] (www.clinicaltrials.gov). Our institute is also conducting a phase I trial studying the side effects and best dose of DIM toward eventual treat-ment of prostate cancer (www.clinicaltrials.gov).
Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) is believed to be the most potent catechin from green tea for the inhibition of oncogenesis and reduction of oxidative stress [114]. EGCG shows strong antioxidant and
anti-NF-κB activity. It has been reported that EGCG treatment inhibits activa-tion of IKK, phosphorylaactiva-tion of IκBα, and translocation of NF-κB to the nucleus [115], demonstrating that EGCG is a potent inhibitor of NF-κB. In rodent models of polymicrobial sepsis, EGCG improves hypoten-sion and survival by inhibiting activation of NF-κB and subsequent NOS gene expression in aortic smooth muscle cells, suggesting that
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
EGCG is a potential nutritional supplement or pharmacologic agent in patients with sepsis [116]. By the inhibition of NF-κB, EGCG also reg-ulates inflammatory response in autoimmune encephalomyelitis [117] and colitis [118]. Moreover, EGCG has beneficial effects on diabetes [119]. Dietary supplementation with EGCG elevates levels of circulat-ing adiponectin in a model of type II diabetes [120]. EGCG also prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice [121].
Growing evidence shows that EGCG inhibits the proliferation of various cancer cells and induces apoptotic processes in cancer cells through inhibition of NF-κB [114,122]. Moreover, EGCG has been found to reduce the levels of matrix metalloproteinases, suppress angiogen-esis, and inhibit invasion and metastasis. Furthermore, it has been reported that EGCG and tamoxifen synergistically induced apoptosis and growth inhibition in cancer cells [123] and this effect of EGCG could be mediated through inhibition of NF-κB. These findings suggest that EGCG is a potent antioxidant that is likely to be useful for cancer prevention and treatment.
An NCI-sponsored phase I/II trial of decaffeinated green tea extracts for patients with asymptomatic, early stage of chronic lymphocytic leukemia has been conducted at the Mayo Clinic to define the optimal dosing, schedule, toxicities, and clinical efficacy [124]. Several phase II trials using green tea in preventing breast, prostate, lung, cervical, and esophageal cancers are being conducted (www.clinicaltrials.gov). A phase II trial of erlotinib and green tea extract in preventing cancer recurrence in former smokers who have undergone surgery for bladder cancer is also being conducted (www.clinicaltrials.gov). In addition, a phase II trial to examine the effect of green tea extract on type II di-abetes and to explore the relationship between green tea extract and related hormone peptides is being conducted (www.clinicaltrials.gov).
Resveratrol
Resveratrol (3,5,4-trihydroxystilbene) is a phytoalexin present in a wide variety of plant species including grapes. Resveratrol has been shown to have beneficial effects on the reduction of oxidative stress and the prevention of heart disease, degenerative diseases, and cancer [125].
Resveratrol has been found to inhibit NF-κB activation, downreg-ulate COX-2, and decrease synthesis and release of proinflammatory mediators, leading to the suppression of inflammatory responses. In this way, resveratrol inhibitsSerratia marcescens–induced acute pneu-monia in rats [126], experimental autoimmune myocarditis [127], in-flammatory arthritis [128], and lung injury caused by sepsis in rats
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
[129]. By regulation of NF-κB, resveratrol also protects against vascu-lar alterations, aging, and Alzheimer’s disease [130, 131].
Experimental studies have shown that resveratrol inhibits the growth of various cancer cells and induces apoptotic cell death [132, 133]. Resveratrol shows its inhibitory effects on the activation of NF-κB [134], suggesting its role as NF-κB inhibitor that could sensitize cancer cells to chemotherapeutic agents. It has been reported that resvera-trol could sensitize TRAIL-induced apoptosis in pancreatic cancer cells [135], suggesting that use of resveratrol may be a novel approach to enhance the efficacy of chemotherapy for cancer treatment.
Several clinical trials using resveratrol in preventing colorectal can-cer, follicular lymphoma, and Alzheimer’s disease are being conducted (www.clinicaltrials.gov).
Inhibition of NF-
κ
B by Synthetic NF-
κ
B Inhibitors
Dehydroxymethylepoxyquinomicin (DHMEQ)
DHMEQ is a novel synthesized NF-κB inhibitor. It has been found that DHMEQ inhibited NF-κB translocation to the nucleus and in-duced apoptotic cell death [136]. DHMED has been reported to suppress diabetes-induced retinal inflammation by inhibiting the angiotensin II type 1 receptor and NF-κB [137]. DHMEQ also suppresses osteoclas-togenesis and expression of NFATc1 in mouse arthritis through the inhibition of NF-κB [138].
It has been found that DHMEQ inhibited the growth and infiltration of cancer cells transplanted in SCID mice [136,139]. More importantly, DHMEQ could inhibit constitutively activated NF-κB and exhibited synergistically inhibitory effect on cancer cell growth with cisplatin [140]. In cancer cells, DHMEQ decreased the level of activated nuclear NF-κB and attenuated NF-κB activation induced by cisplatin. The com-bination of DHMEQ with cisplatin also decreased the levels of IL-6 and Bcl-xL mRNA, suggesting that DHMEQ could inhibit expression of NF-κB target genes [140]. The effect of DHMEQ combined with TNF-α
were evaluated in PK-8 pancreatic cancer cells [141]. The addition of DHMEQ to TNF-αmarkedly induced apoptosis with downregulation of antiapoptotic c-FLIP and survivin in PK-8 cells [141]. These findings suggest that DHMEQ in combination with chemotherapeutic agents may be a promising strategy for the treatment of cancer.
Cyclooxygenase-2 (COX-2) Inhibitors
Aspirin has been used in inflammatory diseases for a long time. In atherosclerosis, low-dose aspirin suppresses vascular inflammation
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
and increases the stability of atherosclerotic plaques, both of which contribute to its antiatherogenic effect [142]. Experimental studies have shown that aspirin inhibits constitutively activated NF-κB in cell culture and, in turn, decreases the expression of the NF-κB down-stream gene, especially COX-2 [143]. Recently, aspirin is also consid-ered as a cancer prevention drug because of its ability to downreg-ulate NF-κB and COX-2. Another COX-2 inhibitor, SC236, has been shown to suppress NF-κB DNA binding activity and NF-κB–mediated gene transcription [144]. It is believed that SC236 directly targets proteins that facilitate the nuclear translocation of NF-κB, thereby suppressing the nuclear translocation of NF-κB [144] by inhibiting COX-2.
Celecoxib, one of the COX-2 inhibitors, has been found to abrogate TNF-α–induced NF-κB activation through inhibition of IKK and Akt in human non–small cell lung carcinoma [145]. We have found that cele-coxib significantly inhibited NF-κB activation and COX-2 expression in pancreatic cancer cells [146, 147]. We and others have also found that celecoxib combined with erlotinib (EGFR blocker) or curcumin syner-gistically potentiate the growth inhibitory and proapoptotic effects in pancreatic cancer cells [146, 148], suggesting that celecoxib could be a potent agent for combination treatment of cancers together with inhi-bition of NF-κB and EGFR.
Several phase II and III clinical trials using celecoxib in combina-tion treatment of pancreatic, prostate, lung, and colon cancers are being conducted (www.clinicaltrials.gov). For inflammation, a phase IV trial is being conducted to determine if celecoxib can reduce the blood ves-sel inflammation associated with high cholesterol and heart disease (www.clinicaltrials.gov). Another phase IV trial is being conducted to determine whether the anti-inflammatory drug celecoxib can delay the onset of Alzheimer’s disease in people with age-associated memory im-pairment (www.clinicaltrials.gov).
Parthenolide
Parthenolide is a synthesized small molecule that suppresses NF-κB activation. It has been found that parthenolide inhibits the activ-ity of IKK and NF-κB and suppresses the inflammatory response in cystic fibrosis cells [149]. Parthenolide also modulates the
NF-κB–mediated inflammatory responses in experimental atherosclerosis [150]. Importantly, parthenolide also sensitizes cancer cells to TNF-α– induced apoptosis by inhibition of NF-κB in human cancer cells [151]. In pancreatic cancer cell lines (BxPC-3, PANC-1, and MIA PaCa-2), parthenolide treatment dose-dependently increased the amount of
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
IκBαand decreased NF-κB DNA binding activity [152]. By the inhi-bition of NF-κB pathway, parthenolide sensitized pancreatic cancer cells to as NSAID. The combination treatment with parthenolide and NSAID sulindac synergistically inhibited cell growth and induced apop-tosis, suggesting that combination treatment with NF-κB inhibitor and NSAIDs could be a novel strategy for the treatment of cancer [152].
Sulfasalazine
Sulfasalazine is commonly used as an anti-inflammatory agent and is known as a potent inhibitor of NF-κB. Sulfasalazine inter-feres with IKK/NF-κB pathway to regulate the release of proin-flammatory cytokines from human adipose tissue and skeletal mus-cle [153]. Muerkoster et al. have investigated whether blockade of NF-κB activity with sulfasalazine is suitable for overcoming NF-κB– induced chemoresistancein vivo in a mouse model of pancreatic can-cer [154]. They found that treatment with chemotherapeutic agent etoposide alone moderately reduced tumor size (32–35% reduction) compared with untreated tumors. Sulfasalazine alone only temporar-ily decreased the tumor sizes. However, sulfasalazine in combination with etoposide significantly reduced tumor size (80% reduction) in all experiments. TUNEL staining showed higher numbers of apoptotic cells in tumors from the combination group. Immunohistochemical staining showed that sulfasalazine treatment abolished the basal
NF-κB activity in tumor xenografts, suggesting that sulfasalazine sensi-tizes cancer cells to chemotherapeutic agents by inhibition of NF-κB [154].
Several clinical trials are being conducted to investigate the ef-fects of sulfasalazine on pediatric juvenile idiopathic arthritis, rheuma-toid arthritis, spondylitis, atherosclerosis, and coronary artery disease (www.clinicaltrials.gov).
Proteasome Inhibitor: PS-341 and MG-132
Proteasome inhibitors PS-341 (bortezomib, Velcade, Millennium Pharmaceuticals, Cambridge, MA) and MG-132 have been known to block intracellular degradation of IκBαproteins and, in turn, inhibit ac-tivation of NF-κB. These proteasome inhibitors have been used as anti-inflammatory drugs [155]. However, these drugs have also been used for cancer treatment. PS-341 has been found to completely abolish NF-κB DNA binding activity through inhibition of IκBαdegradation and to in-hibit expression of Bcl-xL in pancreatic cancer cells [156]. Moreover, PS-341 could also sensitize cancer cells to apoptosis induced by paclitaxel
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
through the inhibition of NF-κB. In addition to PS-341, MG-132 has also been found to inhibit NF-κB activation by downregulation of IκBα
degradation. It has been found that MG-132 also downregulates expres-sion of MMP-9, one of the NF-κB downstream target genes, through in-hibition of NF-κB [157]. These findings suggest that combination treat-ment with proteasome inhibitors could enhance the efficacy of cancer therapies.
Several clinical trials using PS-341 (bortezomib) are being conducted for the combination treatment of pancreatic, breast, prostate, lung, colorectal, and head and neck cancers (www.clinicaltrials.gov).
BAY 11-7082
BAY 11-7082 is a synthetic NF-κB inhibitor that downregulates IκBαphosphorylation and leads to the inhibition of NF-κB activation. BAY 11-7082 interferes with NF-κB pathway to regulate the release of proinflammatory cytokines from human adipose and skeletal muscle cells [153]. BAY 11-7082 also downregulates NF-κB–inducible genes such as IL-10, IL-15, Bcl-xL, TNF-α, and TGF-β [158], suggesting its anti-inflammatory effect. Importantly, BAY 11-7082 inhibits constitu-tive NF-κB activity, leading to cell cycle arrest in G1 phase and rapid
induction of apoptosis in multidrug-resistant cancers [159], suggest-ing that suppression of constitutive NF-κB activity by BAY 11-7082 could be a useful treatment strategy for multidrug-resistant cancers. Moreover, BAY 11-7082 has been found to sensitize cancer cells to TPA-induced growth inhibition and apoptosis through inhibition of NF-κB activation induced by TPA [160], suggesting that BAY 11-7082 could be used to enhance the efficacy of chemotherapy in combination treatment.
CONCLUSION
NF-κB is an important transcription factor that plays central roles in oxidative stress, inflammation, and carcinogenesis. It regulates the ex-pression of genes critically involved in the processes of aging, metabolic diseases, inflammatory diseases, carcinogenesis, cancer cell EMT, inva-sion, and metastasis. Targeting NF-κB may be a novel and important preventive or therapeutic strategy against human cancers and chronic diseases that are caused in part due to induction of oxidative stress. Therefore, the strategies focused on reducing the activity of NF-κB by natural or synthetic NF-κB inhibitors could become a novel and potent approach for enhancing the therapeutic efficacy of conventional agents for the treatment of human cancers and inflammatory diseases.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
REFERENCES
[1] R. Sen and D. Baltimore, Multiple nuclear factors interact with the immunoglob-ulin enhancer sequences.Cell46: 705–716, 1986.
[2] R. Sen and D. Baltimore, Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism.Cell47: 921–928, 1986. [3] M. Karin and F.R. Greten, NF-kappaB: linking inflammation and immunity to
cancer development and progression.Nat Rev Immunol5: 749–759, 2005. [4] P.J. Barnes and M. Karin, Nuclear factor-kappaB: a pivotal transcription factor in
chronic inflammatory diseases.N Engl J Med336: 1066–1071, 1997.
[5] V. Anest, J.L. Hanson, P.C. Cogswell, K.A. Steinbrecher, B.D. Strahl, and A.S. Bald-win, A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression.Nature423: 659–663, 2003.
[6] C. Granet, W. Maslinski, and P. Miossec, Increased AP-1 and NF-kappaB activation and recruitment with the combination of the proinflammatory cytokines IL-1beta, tumor necrosis factor alpha and IL-17 in rheumatoid synoviocytes.Arthritis Res Ther6: R190–R198, 2004.
[7] Y.M. Zhu, D.A. Bradbury, L. Pang, and A.J. Knox, Transcriptional regulation of interleukin (IL)-8 by bradykinin in human airway smooth muscle cells in-volves prostanoid-dependent activation of AP-1 and nuclear factor (NF)-IL-6 and prostanoid-independent activation of NF-kappaB.J Biol Chem278: 29366–29375, 2003.
[8] Y.C. Hseu, F.Y. Wu, J.J. Wu, J.Y. Chen, W.H. Chang, F.J. Lu, Y.C. Lai, and H.L. Yang, Anti-inflammatory potential of Antrodia camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-kappaB pathway.Int Immunopharmacol
5: 1914–1925, 2005.
[9] A.K. Kiemer, T. Hartung, C. Huber, and A.M. Vollmar, Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-kappaB pathway.J Hepatol38: 289–297, 2003.
[10] C. Jobin, O. Morteau, D.S. Han, and S.R. Balfour, Specific NF-kappaB blockade se-lectively inhibits tumour necrosis factor-alpha-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells.Immunology95: 537–543, 1998.
[11] D.J. Van Antwerp, S.J. Martin, T. Kafri, D.R. Green, and I.M. Verma, Suppression of TNF-alpha-induced apoptosis by NF-kappaB.Science274: 787–789, 1996. [12] M. Wu, H. Lee, R.E. Bellas, S.L. Schauer, M. Arsura, D. Katz, M.J. FitzGerald, T.L.
Rothstein, D.H. Sherr, and G.E. Sonenshein, Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells.EMBO J15: 4682–4690, 1996.
[13] G. Helbig, K.W. Christopherson, P. Bhat-Nakshatri, S. Kumar, H. Kishimoto, K.D. Miller, H.E. Broxmeyer, and H. Nakshatri, NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4.J Biol Chem278: 21631–21638, 2003.
[14] S. Huang, C.A. Pettaway, H. Uehara, C.D. Bucana, and I.J. Fidler, Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis.Oncogene20: 4188–4197, 2001. [15] R.S. Samant, D.W. Clark, R.A. Fillmore, M. Cicek, B.J. Metge, K.H. Chandramouli,
A.F. Chambers, G. Casey, D.R. Welch, and L.A. Shevde, Breast cancer metasta-sis suppressor 1 (BRMS1) inhibits osteopontin transcription by abrogating NF-kappaB activation.Mol Cancer6: 6, 2007.
[16] B.R. van den, G.R. Haenen, B.H. van, den, and A. Bast, Transcription factor NF-kappaB as a potential biomarker for oxidative stress.Br J Nutr 86(Suppl 1): S121–S127, 2001.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
[17] J.N. Davis, O. Kucuk, Z. Djuric, and F.H. Sarkar, Soy isoflavone supplementation in healthy men prevents NF-kappa B activation by TNF-alpha in blood lymphocytes. Free Radic Biol Med30: 1293–1302, 2001.
[18] L. Deng, Y.C. Lin-Lee, F.X. Claret, and M.T. Kuo, 2-acetylaminofluorene up-regulates rat mdr1b expression through generating reactive oxygen species that activate NF-kappa B pathway.J Biol Chem276: 413–420, 2001.
[19] D. Galaris and A. Evangelou, The role of oxidative stress in mecha-nisms of metal-induced carcinogenesis. Crit Rev Oncol Hematol 42: 93–103, 2002.
[20] M. Karin, Nuclear factor-kappaB in cancer development and progression.Nature
441: 431–436, 2006.
[21] T. Finkel and N.J. Holbrook, Oxidants, oxidative stress and the biology of ageing. Nature408: 239–247, 2000.
[22] E. Ho and T.M. Bray, Antioxidants, NFkappaB activation, and diabetogenesis.Proc Soc Exp Biol Med222: 205–213, 1999.
[23] S.E. Lamhamedi-Cherradi, S. Zheng, B.A. Hilliard, L. Xu, J. Sun, S. Alsheadat, H.C. Liou, and Y.H. Chen, Transcriptional regulation of type I diabetes by NF-kappa B.J. Immunol171: 4886–4892, 2003.
[24] Z.U. Mollah, S. Pai, C. Moore, B.J. O’Sullivan, M.J. Harrison, J. Peng, K. Phillips, J.B. Prins, J. Cardinal, and R. Thomas, Abnormal NF-{kappa}B function char-acterizes human type 1 diabetes dendritic cells and monocytes.J Immunol180: 3166–3175, 2008.
[25] M.F. Beal, Oxidatively modified proteins in aging and disease.Free Radic Biol Med
32: 797–803, 2002.
[26] B. Kaltschmidt, M. Uherek, B. Volk, P.A. Baeuerle, and C. Kaltschmidt, Transcrip-tion factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci U. S. A94: 2642–2647, 1997.
[27] S. Hunot, B. Brugg, D. Ricard, P.P. Michel, M.P. Muriel, M. Ruberg, B.A. Faucheux, Y. Agid, and E.C. Hirsch, Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease.Proc Natl Acad Sci U. S. A94: 7531–7536, 1997.
[28] R.E. Simmonds and B.M. Foxwell, Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation.Rheumatology (Oxford)
47: 584–590, 2008.
[29] L. Hajra, A.I. Evans, M. Chen, S.J. Hyduk, T. Collins and M.I. Cybulsky. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation.Proc Natl Acad Sci U. S. A.97: 9052–9057, 2000.
[30] H. Isomoto, Y. Mizuta, M. Miyazaki, F. Takeshima, K. Omagari, K. Murase, T. Nishiyama, K. Inoue, I. Murata, and S. Kohno, Implication of NF-kappaB in Helicobacter pylori-associated gastritis.Am. J. Gastroenterol95: 2768–2776, 2000.
[31] S. Schreiber, S. Nikolaus, and J. Hampe, Activation of nuclear factor kappa B inflammatory bowel disease.Gut42: 477–484, 1998.
[32] M.A. Brown and W.K. Jones, NF-kappaB action in sepsis: the innate immune system and the heart.Front. Biosci9: 1201–1217, 2004.
[33] P. Burgos, C. Metz, P. Bull, R. Pincheira, L. Massardo, C. Errazuriz, M.R. Bono, S. Jacobelli, and A. Gonzalez, Increased expression of c-rel, from the NF-kappaB/Rel family, in T cells from patients with systemic lupus erythematosus.J Rheumatol
27: 116–127, 2000.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
[34] K. Bauerova and A. Bezek, Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis.Gen Physiol Biophys18(Spec No): 15– 20, 1999.
[35] M. Yoshizumi, K. Tsuchiya, and T.Tamaki, Signal transduction of reactive oxygen species and mitogen-activated protein kinases in cardiovascular disease.J Med Invest48: 11–24, 2001.
[36] Y. Yamamoto and R.B. Gaynor, Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer.J Clin Invest107: 135–142, 2001.
[37] A.C. Bharti and B.B. Aggarwal, Nuclear factor-kappa B and cancer: its role in prevention and therapy.Biochem Pharmacol64: 883–888, 2002.
[38] H. Algul, M. Treiber, M. Lesina, and R.M. Schmid, Mechanisms of dis-ease: chronic inflammation and cancer in the pancreas—a potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol 4: 454–462, 2007.
[39] H.S. Perwez and C.C. Harris, Inflammation and cancer: an ancient link with novel potentials.Int J Cancer121: 2373–2380, 2007.
[40] R.C. Bargou, F. Emmerich, D. Krappmann, K. Bommert, M.Y. Mapara, W. Arnold, H.D. Royer, E. Grinstein, A. Greiner, C. Scheidereit, and B. Dorken, Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest 100: 2961–2969, 1997.
[41] T. Hideshima, D. Chauhan, P. Richardson, C. Mitsiades, N. Mitsiades, T. Hayashi, N. Munshi, L. Dang, A. Castro, V. Palombella, J. Adams, and K.C. Anderson, NF-kappa B as a therapeutic target in multiple myeloma,J Biol Chem277: 16639-16647, 2002.
[42] H. Nakshatri, P. Bhat-Nakshatri, D.A. Martin, R.J. Goulet, Jr., and G.W.Sledge, Jr., Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth.Mol Cell Biol17: 3629–3639, 1997.
[43] S. Shukla, G.T. MacLennan, P. Fu, J. Patel, S.R. Marengo, M.I. Resnick, and S.Gupta, Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression.Neoplasia6: 390–400, 2004.
[44] L. Li, B.B. Aggarwal, S. Shishodia, J. Abbruzzese, and R.Kurzrock, Nuclear factor-kappaB and Ifactor-kappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis.Cancer101: 2351– 2362, 2004.
[45] B. Holcomb, M. Yip-Schneider, and C.M.Schmidt, The role of nuclear factor kappaB in pancreatic cancer and the clinical applications of targeted therapy.Pancreas36: 225–235, 2008.
[46] G. Levidou, P. Korkolopoulou, N. Nikiteas, N. Tzanakis, I. Thymara, A.A. Saetta, C. Tsigris, G. Rallis, K. Vlasis, and E.Patsouris, Expression of nuclear factor kap-paB in human gastric carcinoma: relationship with I kapkap-paB a and prognostic significance.Virchows Arch450: 519–527, 2007.
[47] C.T. Allen, J.L. Ricker, Z. Chen, and W.C.Van, Role of activated nuclear factor-kappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neckHead Neck29: 959–971, 2007.
[48] S. Liptay, C.K. Weber, L. Ludwig, M. Wagner, G. Adler, and R.M. Schmid, Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer105: 735–746, 2003.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
[49] S. Fujioka, G. M.Sclabas, C. Schmidt, W.A. Frederick, Q.G. Dong, J.L. Abbruzzese, D.B. Evans, C. Baker, and P.J. Chiao, Function of nuclear factor kappaB in pan-creatic cancer metastasis.Clin Cancer Res9: 346–354, 2003.
[50] S. Fujioka, G.M. Sclabas, C. Schmidt, J. Niu, W.A. Frederick, Q.G. Dong, J.L. Abbruzzese, D.B. Evans, C. Baker, and P.J. Chiao, Inhibition of constitutive NF-kappa B activity by I NF-kappa B alpha M suppresses tumorigenesis.Oncogene22: 1365–1370, 2003.
[51] W. Wang, J.L. Abbruzzese, D.B. Evans, and P.J. Chiao, Overexpression of urokinase-type plasminogen activator in pancreatic adenocarcinoma is regulated by constitutively activated RelA.Oncogene18: 4554–4563, 1999.
[52] S.E. Chuang, P.Y. Yeh, Y.S. Lu, G.M. Lai, C.M. Liao, M. Gao, and A.L. Cheng, Basal levels and patterns of anticancer drug-induced activation of nuclear factor-kappaB (NF-kappaB), and its attenuation by tamoxifen, dexamethasone, and curcumin in carcinoma cells.Biochem Pharmacol63: 1709–1716, 2002.
[53] Y. Li, F. Ahmed, S. Ali, P.A. Philip, O. Kucuk, and F.H. Sarkar, Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apop-tosis induced by chemotherapeutic agents in human cancer cells.Cancer Res65: 6934–6942, 2005.
[54] P.Y. Yeh, S.E. Chuang, K.H. Yeh, Y.C. Song, C.K. Ea, and A.L. Cheng, In-crease of the resistance of human cervical carcinoma cells to cisplatin by inhi-bition of the MEK to ERK signaling pathway partly via enhancement of anti-cancer drug-induced NF kappa B activation.Biochem Pharmacol63: 1423–1430, 2002.
[55] A. Arlt, J. Vorndamm, S. Muerkoster, H. Yu, W.E. Schmidt, U.R. Folsch, and H.Schafer, Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res62: 910–916, 2002.
[56] S. Muerkoster, A. Arlt, B. Sipos, M. Witt, M. Grossmann, G. Kloppel, H. Kalthoff, U.R. Folsch, and H.Schafer, Increased expression of the E3-ubiquitin ligase recep-tor subunit betaTRCP1 relates to constitutive nuclear facrecep-tor-kappaB activation and chemoresistance in pancreatic carcinoma cells.Cancer Res65: 1316–1324, 2005.
[57] J.P. Thiery, Epithelial-mesenchymal transitions in tumour progression.Nat. Rev. Cancer2: 442–454, 2002.
[58] T. Brabletz, A. Jung, S. Reu, M. Porzner, F. Hlubek, L.A. Kunz-Schughart, R. Knuechel, and T. Kirchner, Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment.Proc Natl Acad Sci U. S. A98: 10356–10361, 2001.
[59] V. Stemmer, C.B. de, G. Berx, and J.Behrens, Snail promotes Wnt target gene expression and interacts with beta-catenin.Oncogene2008.
[60] T.R. Graham, H.E. Zhau, V.A. Odero-Marah, A.O. Osunkoya, K.S. Kimbro, M. Tighiouart, T. Liu, J.W. Simons, and R.M. O’Regan, Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells.Cancer Res68: 2479–2488, 2008.
[61] M.H. Yang, M.Z. Wu, S.H. Chiou, P.M. Chen, S.Y. Chang, C.J. Liu, S.C. Teng, and K.J.Wu, Direct regulation of TWIST by HIF-1alpha promotes metastasis.Nat Cell Biol10: 295–305, 2008.
[62] S. Spaderna, O. Schmalhofer, M. Wahlbuhl, A. Dimmler, K. Bauer, A. Sultan, F. Hlubek, A. Jung, D. Strand, A. Eger, T. Kirchner, J. Behrens, and T.Brabletz, The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer.Cancer Res68: 537–544, 2008.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
[63] J. Yang, S.A. Mani, J.L. Donaher, S. Ramaswamy, R.A. Itzykson, C. Come, P. Sav-agner, I. Gitelman, A. Richardson, and R.A. Weinberg. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis.Cell117: 927–939, 2004.
[64] T. Muller, G. Bain, X. Wang, and J. Papkoff, Regulation of epithelial cell migration and tumor formation by beta-catenin signaling.Exp Cell Res280: 119–133, 2002. [65] C. Min, S.F. Eddy, D.H. Sherr, and G.E. Sonenshein, NF-kappaB and epithelial to
mesenchymal transition of cancer.J Cell Biochem2008.
[66] D.C. Radisky and M.J. Bissell, NF-kappaB links oestrogen receptor signalling and EMT.Nat Cell Biol9: 361–363, 2007.
[67] H.L. Chua, P. Bhat-Nakshatri, S.E. Clare, A. Morimiya, S. Badve, and H. Naksha-tri, NF-kappaB represses E-cadherin expression and enhances epithelial to mes-enchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2.Oncogene26: 711–724, 2007.
[68] S. Julien, I. Puig, E. Caretti, J. Bonaventure, L. Nelles, R.F. van, C. Dargemont, A.G. de Herreros, A. Bellacosa, and L.Larue, Activation of NF-kappaB by Akt up-regulates Snail expression and induces epithelium mesenchyme transition. Onco-gene26: 7445–7456, 2007.
[69] X. Wang, K. Belguise, N. Kersual, K.H. Kirsch, N.D. Mineva, F. Galtier, D. Chal-bos, and G.E. Sonenshein, Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2.Nat Cell Biol9: 470–478, 2007.
[70] D. Kong, Z. Wang, S.H. Sarkar, Y. Li, S. Banerjee, A. Saliganan, H.R. Kim, M.L. Cher, and F.H. Sarkar, PDGF-D over-expression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells.Stem Cells26: 1425–1435, 2008.
[71] C.H. Chung, J.S. Parker, K. Ely, J. Carter, Y. Yi, B.A. Murphy, K.K. Ang, A.K. El-Naggar, A.M. Zanation, A.J. Cmelak, S. Levy, R.J. Slebos, and W.G. Yarbrough, Gene expression profiles identify epithelial-to-mesenchymal transition and acti-vation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma.Cancer Res66: 8210–8218, 2006.
[72] M. Zhang, X. Xie, A.H. Lee, and C.W. Binns, Soy and isoflavone intake are asso-ciated with reduced risk of ovarian cancer in southeast China.Nutr Cancer49: 125–130, 2004.
[73] A.H. Wu, M.C. Yu, C.C. Tseng, N.C. Twaddle, and D.R. Doerge, Plasma isoflavone levels versus self-reported soy isoflavone levels in Asian-American women in Los Angeles County.Carcinogenesis25: 77–81, 2004.
[74] M. Hamalainen, R. Nieminen, P. Vuorela, M. Heinonen and E. Moilanen. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, nargenin, and pelargonidin inhibit only NF-kappaB activation along with their in-hibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm2007: 45673, 2007.
[75] J.L. Kang, H.W. Lee, H.S. Lee, I.S. Pack, Y. Chong, V. Castranova, and Y. Koh, Genistein prevents nuclear factor-kappa B activation and acute lung injury in-duced by lipopolysaccharide.Am J Respir Crit Care Med164: 2206–2212, 2001. [76] R. Kalhan, L.J. Smith, M.C. Nlend, A. Nair, J.L. Hixon, and P.H. Sporn, A
mecha-nism of benefit of soy genistein in asthma: inhibition of eosinophil p38-dependent leukotriene synthesis.Clin Exp Allergy38: 103–112, 2008.
[77] L.J. Smith, J.T. Holbrook, R. Wise, M. Blumenthal, A.J. Dozor, J. Mastronarde, and L. Williams, Dietary intake of soy genistein is associated with lung function in patients with asthma.J Asthma41: 833–843, 2004.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
[78] S. Banerjee, Y. Zhang, S. Ali, M. Bhuiyan, Z. Wang, P.J. Chiao, P.A. Philip, J. Abbruzzese, and F.H. Sarkar, Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer.Cancer Res65: 9064–9072, 2005.
[79] Y. Li and F.H. Sarkar, Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway.Clin Cancer Res8: 2369–2377, 2002.
[80] J.J. Raffoul, S. Banerjee, V. Singh-Gupta, Z.E. Knoll, A. Fite, H. Zhang, J. Abrams, F.H. Sarkar, and G.G. Hillman, Down-regulation of apurinic/apyrimidinic endonu-clease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo.Cancer Res67: 2141–2149, 2007.
[81] C.H. Takimoto, K. Glover, X. Huang, S.A. Hayes, L. Gallot, M. Quinn, B.D. Jo-vanovic, A. Shapiro, L. Hernandez, A. Goetz, V. Llorens, R. Lieberman, J.A. Crow-ell, B.A. Poisson, and R.C. Bergan, Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomarkers Prev12: 1213–1221, 2003.
[82] M. Banerjee, L.M. Tripathi, V.M. Srivastava, A. Puri, and R. Shukla, Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat.Immunopharmacol Immunotoxicol25: 213–224, 2003. [83] C.V. Rao, A. Rivenson, B. Simi, and B.S. Reddy, Chemoprevention of colon
car-cinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res55: 259–266, 1995.
[84] A.C. Bharti, N. Donato, S. Singh, and B.B. Aggarwal. Curcumin (diferuloyl-methane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppres-sion of proliferation and induction of apoptosis.Blood101: 1053–1062, 2003. [85] B.B. Aggarwal and S. Shishodia, Molecular targets of dietary agents for prevention
and therapy of cancer.Biochem Pharmacol71: 1397–1421, 2006.
[86] S.E. Chuang, A.L. Cheng, J.K. Lin, and M.L. Kuo, Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats.Food Chem Toxicol38: 991–995, 2000.
[87] K. Sugimoto, H. Hanai, K. Tozawa, T. Aoshi, M. Uchijima, T. Nagata, and Y. Koide, Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice.Gastroenterology123: 1912–1922, 2002.
[88] I.A. Leclercq, G.C. Farrell, C. Sempoux, P.A. dela, and Y. Horsmans, Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steato-hepatitis in mice.J Hepatol41: 926–934, 2004.
[89] Y.S. Kim, Y. Ahn, M.H. Hong, S.Y. Joo, K.H. Kim, I.S. Sohn, H.W. Park, Y.J. Hong, J.H. Kim, W. Kim, M.H. Jeong, J.G. Cho, J.C. Park, and J.C. Kang, Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells.J Cardiovasc Pharmacol50: 41–49, 2007.
[90] K. Reyes-Gordillo, J. Segovia, M. Shibayama, P. Vergara, M.G. Moreno, and P. Muriel, Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress.Biochim Biophys Acta1770: 989–996, 2007.
[91] S.P. Weisberg, R. Leibel, and D.V. Tortoriello. Dietary curcumin significantly im-proves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology149: 3549–3558, 2008.
[92] A. Duvoix, F. Morceau, S. Delhalle, M. Schmitz, M. Schnekenburger, M.M. Galteau, M. Dicato, and M. Diederich, Induction of apoptosis by curcumin: mediation by
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
glutathione S-transferase P1-1 inhibition.Biochem Pharmacol 66: 1475–1483, 2003.
[93] Z. Wang, Y. Zhang, S. Banerjee, Y. Li, and F.H. Sarkar, Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells.Cancer 106: 2503–2513, 2006.
[94] B.E. Bachmeier, I.V. Mohrenz, V. Mirisola, E. Schleicher, F. Romeo, C. Hohneke, M. Jochum, A.G. Nerlich, and U. Pfeffer, Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB.Carcinogenesis29: 779–789, 2008.
[95] Y.G. Lin, A.B. Kunnumakkara, A. Nair, W.M. Merritt, L.Y. Han, G.N. rmaiz-Pena, A.A. Kamat, W.A. Spannuth, D.M. Gershenson, S.K. Lutgendorf, B.B. Aggarwal, and A.K. Sood, Curcumin inhibits tumor growth and angiogenesis in ovarian carci-noma by targeting the nuclear factor-kappaB pathway.Clin Cancer Res13: 3423– 3430, 2007.
[96] S. Aggarwal, Y. Takada, S. Singh, J.N. Myers, and B.B. Aggarwal. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling.Int J Cancer111: 679–692, 2004.
[97] S.M. Plummer, K.A. Holloway, M.M. Manson, R.J. Munks, A. Kaptein, S. Farrow, and L.Howells, Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex.Oncogene18: 6013–6020, 1999.
[98] B.B. Aggarwal, S. Shishodia, Y. Takada, S. Banerjee, R.A. Newman, C.E. Bueso-Ramos, and J.E. Price, Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice.Clin Cancer Res11: 7490–7498, 2005.
[99] R.A. Sharma, S.A. Euden, S.L. Platton, D.N. Cooke, A. Shafayat, H.R. He-witt, T.H. Marczylo, B. Morgan, D. Hemingway, S.M. Plummer, M. Pirmo-hamed, A.J. Gescher, and W.P. Steward, Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance.Clin Cancer Res10: 6847–6854, 2004.
[100] A.L. Cheng, C.H. Hsu, J.K. Lin, M.M. Hsu, Y.F. Ho, T.S. Shen, J.Y. Ko, J.T. Lin, B.R. Lin, W. Ming-Shiang, H.S. Yu, S.H. Jee, G.S. Chen, T.M. Chen, C.A. Chen, M.K. Lai, Y.S. Pu, M.H. Pan, Y.J. Wang, C.C. Tsai, and C.Y. Hsieh, Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions.Anticancer Res21: 2895–2900, 2001.
[101] N. Dhillon, B.B. Aggarwal, R.A. Newman, R.A. Wolff, A.B. Kunnumakkara, J.L. Abbruzzese, C.S. Ng, V. Badmaev, R. Kurzrock, Phase II trial of curcumin in patients with advanced pancreatic cancer.Clin Cancer Res 14: 4491–4499, 2008.
[102] L. Li, B. Ahmed, K. Mehta, and R. Kurzrock, Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther6: 1276–1282, 2007.
[103] L. Li, F.S. Braiteh, and R.Kurzrock, Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis.Cancer104: 1322–1331, 2005.
[104] C.W. Nho and E.Jeffery, Crambene, a bioactive nitrile derived from glucosinolate hydrolysis, acts via the antioxidant response element to upregulate quinone re-ductase alone or synergistically with indole-3-carbinol.Toxicol Appl Pharmacol
198: 40–48, 2004.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI
[105] S.H. Benabadji, R. Wen, J.B. Zheng, X.C. Dong, and S.G. Yuan, Anticarcinogenic and antioxidant activity of diindolylmethane derivatives.Acta Pharmacol Sin25: 666–671, 2004.
[106] H.J. Cho, M.R. Seon, Y.M. Lee, J. Kim, J.K. Kim, S.G. Kim, and J.H. Park, 3,3 -Diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages.J Nutr138: 17–23, 2008.
[107] D.J. Kim, D.H. Shin, B. Ahn, J.S. Kang, K.T. Nam, C.B. Park, C.K. Kim, J.T. Hong, Y.B. Kim, Y.W. Yun, D.D. Jang, and K.H. Yang, Chemoprevention of colon cancer by Korean food plant components.Mutat Res523–524: 99–107, 2003.
[108] Y. Li, S.R. Chinni, and F.H. Sarkar, Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells.Front Biosci10: 236–243, 2005.
[109] K.W. Rahman and F.H. Sarkar, Inhibition of nuclear translocation of nuclear factor-kappaB contributes to 3,3’-diindolylmethane-induced apoptosis in breast cancer cells.Cancer Res65: 364–371, 2005.
[110] C.M. Cover, S.J. Hsieh, E.J. Cram, C. Hong, J.E. Riby, L.F. Bjeldanes, and G.L. Firestone, Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells.Cancer Res59: 1244–1251, 1999.
[111] F.H. Sarkar and Y. Li. Indole-3-carbinol and prostate cancer.J Nutr134: 3493S-3498S, 2004.
[112] R. Naik, S. Nixon, A. Lopes, K. Godfrey, M.H. Hatem, and J.M. Monaghan, A randomized phase II trial of indole-3-carbinol in the treatment of vulvar intraep-ithelial neoplasia.Int J Gynecol Cancer16: 786–790, 2006.
[113] G.A. Reed, K.S. Peterson, H.J. Smith, J.C. Gray, D.K. Sullivan, M.S. Mayo, J.A. Crowell, and A. Hurwitz, A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev 14: 1953–1960, 2005.
[114] H. Mukhtar and N. Ahmad. Green tea in chemoprevention of cancer.Toxicol Sci
52: 111–117, 1999.
[115] F. Afaq, V.M. Adhami, N. Ahmad, and H. Mukhtar, Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal ker-atinocytes by green tea Constituent (-)-epigallocatechin-3-gallate.Oncogene22: 1035–1044, 2003.
[116] D.S. Wheeler, P.M. Lahni, P.W. Hake, A.G. Denenberg, H.R. Wong, C. Snead, J.D. Catravas, and B. Zingarelli, The green tea polyphenol epigallocatechin-3-gallate improves systemic hemodynamics and survival in rodent models of polymicrobial sepsis.Shock28: 353–359, 2007.
[117] O. Aktas, T. Prozorovski, A. Smorodchenko, N.E. Savaskan, R. Lauster, P.M. Kloet-zel, C. Infante-Duarte, S. Brocke, and F.Zipp, Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoim-mune encephalomyelitis.J Immunol173: 5794–5800, 2004.
[118] P.A. Abboud, P.W. Hake, T.J. Burroughs, K. Odoms, M. O’Connor, P. Mangeshkar, H.R. Wong, and B. Zingarelli, Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis.Eur J Pharmacol579: 411–417, 2008.
[119] S.I. Rizvi, M.A. Zaid, R. Anis, and N. Mishra, Protective role of tea catechins against oxidation-induced damage of type 2 diabetic erythrocytes.Clin. Exp. Phar-macol. Physiol32: 70–75, 2005.
[120] M. Shimada, K. Mochizuki, N. Sakurai, and T. Goda, Dietary supplementation with epigallocatechin gallate elevates levels of circulating adiponectin in non-obese type-2 diabetic Goto-Kakizaki rats.Biosci Biotechnol Biochem 71: 2079– 2082, 2007.
Int Rev Immunol Downloaded from informahealthcare.com by HINARI