Response of tomato plants to water stress and calcium nutrition
Texto completo
(2) Agradecimientos. Al Laboratorio de Nutrición Frutal de la Facultad de Agronomía e Ingeniería Forestal de la. Pontificia Universidad Católica por permitirme realizar mi trabajo de. investigación.. A mi profesora guía Claudia Bonomelli, quién fue un pilar fundamental en el desarrollo de este proyecto ya que siempre estuvo apoyándome y guiándome tanto en el ámbito académico como emocional. A mis profesores informantes Pilar Gil, Samuel Contreras y Alvaro Anchondo por su apoyo y contribución a este estudio.. A mis amigas de magister Massiel, Paola Belén y Dani por hacer más fácil todo este proceso y llenarlo de risas en momentos de máximo colapso mental.. Finalmente a mis amigos de la vida, Joaquín, Roberto, Felipe, Belén, Josefina y Consuelo; a mi mamá, mi abuela y Aldo por quererme siempre y soportarme en mis momentos de estrés.. 2.
(3) Al universo…. 3.
(4) Index Abstract................................................................................................................... 5 Introduction............................................................................................................. 6 Materials and methods .......................................................................................... 7 Experimental setup ............................................................................................ 7 Statistical analysis ........................................................................................... 10 Results .................................................................................................................. 10 Water stress, photosynthesis and stomatal conductance ........................... 10 Biomass ............................................................................................................ 13 Fruit parameters............................................................................................... 14 Blossom end rot development ........................................................................ 16 Calcium fractions ............................................................................................. 18 Discussion ............................................................................................................ 19 Conclusions .......................................................................................................... 23 References ........................................................................................................... 24. 4.
(5) RESPONSE OF TOMATO PLANTS TO WATER STRESS AND CALCIUM NUTRITION. María Ignacia Arias Flores. Pontificia Universidad Católica de Chile. Abstract Calcium is widely accepted as the main factor responsible for blossom-end rot appearance in tomato fruit. However, there is no clear evidences of how this physiological disorder is trigger. In this study, tomato plants from hybrid cultivar H8504 were used to make a factorial arrangement with two factors, water and calcium, being a total of four treatments: water stress and without calcium supply; water stress and calcium supply; optimum watering and without calcium supply; and optimum watering and calcium supply. The results of biomass, yield, fruit quality and nutrient content changed with water stress treatments; only the standard germination, was not affected. In conclusion according to these results, although the water factor is the main responsible for the development of fruits with Blossom-end rot, the combined water stress and without calcium supply treatment was the most unfavorable condition for the plant. This treatment also showed predominance of protoxylem traqueary elements, and a less developed xylem tissue compared to plants in treatment with optimum watering and calcium supply. This could indicate that the lack of calcium combined with less movement of sap in the xylem, derived from a water deficit, can affect the formation of xylem tissue during the development of the fruit. This difficult the movement of calcium towards the fruit by the xylem and according to this, blossom-end rot could be a primary consequence of an anatomical problem, and that can be affected the distribution of calcium within the damage fruit, healthy fruits have more soluble calcium than fruits that present this physiological disorder.. Key words: Blossom end rot, xylem, mineral nutrition, calcium fractions, Scanning electron microscope.. 5.
(6) Introduction Calcium-dependent physiological disorders are a widespread crop problem at the national and international level; these disorders have puzzled researchers for more than 100 years, and still, much is unknown about the mechanisms involved (Ferguson and Watkins 1989; Saure 2001; Taylor and Locascio 2004). Calcium deficit can affect different organs of the plant, e.g. leaves (tipburn in lettuce, blackheart on celery), flowers (tipburn in artichoke) and fruits (blossom end rot in tomato) (White and Broadley, 2003). Particularly in fruits, two of the most common disorders are Bitter Pit (BP) in apples (Kalcsits, 2016) and Blossom End Rot (BER) in tomato. Since Lyon et al. (1942) and Raleigh and Chucka (1944) found a correlation between the occurrence of BER and Ca 2+ nutrition, BER is now generally attributed to an inadequate level of Ca2+ in the fruits and it is therefore called a “Calcium-related disorder” (Shear, 1975). As stated by Saure (2014), BER disorder “can be triggered by mechanisms that reduce: (1) plant Ca2+ uptake from the soil, (2) fruit Ca2+ uptake from the plant, and (3) Ca2+ translocation within the fruit. These factors will result in an abnormal accumulation and partitioning of Ca 2+ in the cells”. Physiological disorders are not linked to pathogenic microorganisms; instead, they are usually caused by inappropriate living environment, such as poor soil physical and chemical properties, excess or insufficient nutrition, and poor climatic conditions such as high relative humidity (Cornell, 2015; Mi, 2012). Several Ca-deficiency disorders occur in horticulture when calcium is temporarily unavailable in developing fruit tissues. This can occur when Ca 2+ cannot be mobilized from older vegetative tissues and redistributed via the phloem. Therefore, the immediate supply of calcium is exclusively in the xylem, which in turn depends on unidirectional water transport and transpiration stream (White and Broadley, 2003; Bonomelli and Ruiz, 2010). Water availability is key for a proper xylematic calcium transport (Ho and White, 2005). Thus, osmotic stress such as drought or high salt present in the soil can directly affect calcium transport and its distribution throughout the plant. This is due to the changes in the sink capability of the vegetative tissues, the link between water and calcium transport is particularly apparent when examining sink organs with relatively low transpiration rates, such as those that typically occur in fruit (Hocking et al., 2016). At fruitset the transpiration rate of fruit is at its highest, but this quickly declines to almost a tenth of this value later in development, whereas leaf transpiration is maintained high (Hocking et al., 2016).. 6.
(7) Additionally, nutrient deficit and abiotic stresses will both induce a strong hormonal response in the plant in order to adapt to the new restricted conditions. Blossom end rot (BER) is a common physiological disorder in tomato that causes large economic losses in greenhouse- or field-grown tomatoes (Suzuki et al., 2003). BER can produce loss yield of up to 50% of the production worldwide (Taylor and Locascio, 2004). Tomatoes suffering from the disorder often shed early and have unacceptable quality (Zhang, 2003). BER usually starts around umbilicus, with the tissue becoming soaked dark green; after about a week, that part of fruit becomes dark brown and rotten (Dong et al., 2001). One of the first visual symptoms is membrane rupture (Suzuki et al., 2003); it should be borne in mind that relatively poor supply of calcium, which has an important role in the stability of the plasma membrane as well as cell wall (White and Broadley, 2003), is frequently associated with blossom end-rot in tomato (Shao et al., 2018).. However, despite all that has been studied about this disorder, the relative importance or interaction between the lack of water and calcium in the development of this problem is still not well understood. Therefore, the main objectives of this study was to test the influence of water stress and calcium nutrition on the incidence of blossom end rot in tomatoes, and to study the distribution of calcium in the fruit and the morphology of both the fruit cell and the peduncle, due to its relationship with the movement of calcium to the growing sink.. Materials and methods Experimental setup The trial was carried out at the facilities of the Pontificia Universidad Católica de Chile (Santiago, 33°29' S, 70°36' W), during a period of 5 months (October - March 2016). Tomato plants from hybrid cultivar H-8504 (H.J. Heinz Company) were used. Tomato seedlings (having two to three true leaves) were transplanted in 5-liter pots filled with a homogeneous mixture of peat (nutritional composition in Table 1) and perlite (2:1). A drip irrigation system was implemented, with one 2 L‧h-1 autocompensated emitters per pot. Distilled water was used for irrigation to control the delivery of nutrients. There were two irrigation conditions according to the. 7.
(8) treatments, and the irrigation time was varying throughout the development of the plants.. The experimental design was a factorial arrangement with two factors, water and calcium, being a total of four treatments: -W-Ca, water stress and without calcium supply; -W+Ca, water stress and calcium supply; +W-Ca, optimum watering and without calcium supply; +W+Ca, optimum watering and calcium supply. The treatments with water deficit began in fruit set, before this period all the plants were irrigated with the same frequency. The experimental unit comprised five tomato plants, and each of the four treatments had four replicates, for a total of eighty plants.. Table 1. Chemical composition of peat (% and mg/kg). Chemical analysis was made in the Agroanalysis Laboratory in the Pontificia Universidad Católica de Chile.. Element. Value. N. 0.94%. P. 0.11%. K. 0.32%. Ca. 4.03%. Mg. 0.19%. Na. 288 mg/kg. Cu. 37 mg/kg. Fe. 1300 mg/kg. Mn. 57 mg/kg. Zn. 18 mg/kg. B. 12 mg/kg. The plants were fertilized every 15 days manually with 1 liter of nutrient solution following Hogland’s description (Hoagland and Arnon, 1950), all plants received the same nutrients except for the calcium that was applied differently according to the. 8.
(9) treatments, where treatments -W+Ca and +W+Ca received calcium fertilization (200 ppm), while treatments -W-Ca and +W-Ca did not receive calcium fertilization.. Physiological, biomass, fruit quality, seed germination and mineral measurements. During the experiment, net photosynthesis rate (Pn) and stomatal conductance (gs) were measured with a handheld photosynthesis system (CI-340, CID Bio-Science) every 15 days from the onset of water stress treatments. Pn rates and gs were obtained by calculations made by the computing system of the IRGA, based on equations of Leuning and Sands (1989) and expressed as µmol CO 2 m-2 s-1 and mmol H2O m-2 s-1, respectively. Stem water potential (SWP) was measured twice with a pressure chamber at flowering (before water stress began) and fruit set (after water stress initiation) as described by Scholander et al. (1964).. To observe xylem histological and morphological differences between -W-Ca and +W+Ca treatments, cuts of both, fruits and their pedicels, were made with a scalpel, and then observed under scanning electron microscope (FEI, Quanta 250). Plant fresh biomass distribution (roots, shoots, leaves and fruits) was measured at the end of the experiment. Fruit production and quality were characterized by yield (total fresh weight of fruits by plant), equatorial diameter of fruits (cm), and sugar content (°Brix) with a digital refractometer (PAL-1, Atago).. Seeds of several fruits from each treatment were extracted and fermented during 3 days at 20ºC, then washed and dried with air at 30ºC. Once dried, seeds were stored at room temperature in paper bags until evaluation. Seed standard germination was evaluated in 200 seeds from each treatment. Fifty seeds were placed in each of four Petri dishes per treatment, placed among three layers of filter paper saturated with distilled water, then placed in a germination chamber at 20/30ºC (16/8 h). Standard germination percentage was measured by counting normal seedlings at 5 and 14 days after sowing (ISTA, 2011).. Mineral composition analyses (total nitrogen, phosphorus, potassium, magnesium and calcium) at the end of the experiment were done for leaves, stems, roots and. 9.
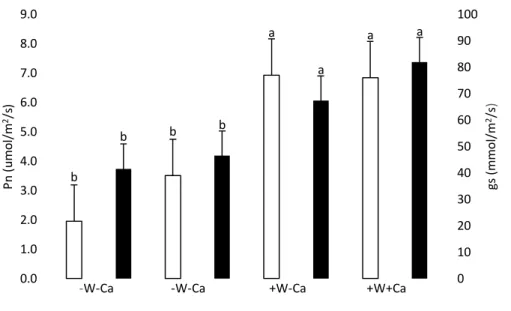
(10) fruits (only the analysis of the last organs are reported here). Vegetal samples were oven-dried for 48 h at 65ºC to constant weight. Cation concentrations were determinated by dry combustion at 500°C until the organic components were converted to ash. The ashed tissue samples were then dissolved in HCl (2M) and concentrations were determined with an atomic absorption spectrophotometer (Varian SpectraAA 220 FS, Varian Techtron Pty. Limited, Victoria, Australia). N and C concentration was determined with a LECO CNS-2000 Macro Elemental Analyzer (Leco, Michigan, USA) (Soltanpour et al., 1982) as well as calcium fractions analysis present in the fruits described by Bonomelli et al. (2018).. Statistical analysis. The data were statistically analyzed with two-way analysis of variance (ANOVA) with water and calcium factors, using STATISTICA DELL (Version 7, Data Analysis and Statistical Software, Dell, Victoria, Australia).. The differences between. treatments were evaluated with Tukey’s test at P < 0.05. Standard germination values from seeds of each treatment were compared using confidence intervals (95%) obtained according to an analysis of proportions (Mead et al., 1993).. Results Water stress, photosynthesis and stomatal conductance To determine the level of water stress caused by treatments, stem water potential (SWP), net photosynthesis rate (Pn) and stomatal conductance (gs) were measured. No significant differences in SWP (p = 0.38) were found at the stage of flowering as expected, since at that time water with holding treatments just began to be applied. On the other hand, at the stage of fruit set, significant differences were found between treatments having different watering treatments (water factor p < 0.001). Treatments with water stress averaged less than -1 MPa of SWP, while treatments without water stress averaged values around -0.6 MPa (Fig 1). In the case of Pn and gs, the same pattern was found, as both physiological parameters were lower in water stress treatments (-W-Ca and -W+Ca) than in optimum water regime (+W-Ca and +W+Ca) (Pn: p water = 0.001, p Ca = 0.52, p water x Ca =. 10.
(11) 0.47) (gs: p water = 0.001, p Ca = 0.25, p water x Ca = 0.57). There were no interactions between water and Ca for any of these parameters in any of the developmental stages.. To evaluate changes in xylem anatomy and morphology caused by the effects of treatments, cuts were made in the fruit pedicel and were analysed by scanning electron microscope (SEM) (Fig. 3).. Stem water potential (MPa). 0 -0.2 -0.4. -0.6 a -0.8. a. a. a b. b. -1 -1.2 -1.4. a. -W-Ca. a. -W-Ca Flowering. +W-Ca. +W+Ca. Fruit set. Figure 1. Stem water potential of water stressed and calcium-deprived plants (-WCa); water-stressed but calcium supplied plants (-W+Ca); optimum watering and without calcium supply (+W-Ca); optimum watering and calcium supply (+W+Ca) treatments in two development stages of tomato plants (flowering and fruit set) growing in pots. On each moment of evaluation, means with different letters indicate significant differences (Tukey test, p < 0.05), bars indicate standard error (n=4).. 11.
(12) 9.0. 100 a. 8.0. 5.0. b. b. 60. b. 50. 4.0. 40. b. 30. 2.0. 20. 1.0. 10. 0.0. gs (mmol/m2/s). 70. 6.0. 3.0. 90. 80. a. 7.0 Pn (umol/m2/s). a. a. -W-Ca. -W-Ca. +W-Ca. +W+Ca. 0. Figure 2. Net photosynthesis (Pn, open bars) and stomatal conductance (gs, filled bars) of water stress and without calcium supply (-W-Ca); water stress and calcium supply (-W+Ca); optimum watering and without calcium supply (+W-Ca); optimum watering and calcium supply (+W+Ca) treatments of pot-grown tomato plants. Means with different letters indicate significant differences (Tukey test, p < 0.05), bars indicate standard error (n=4).. Figure 3. Scanning electron microscopy images of fruit pedicle cuts of optimum watering and calcium supply (a) and water stress and without calcium supply (b) treated tomato plants.. 12.
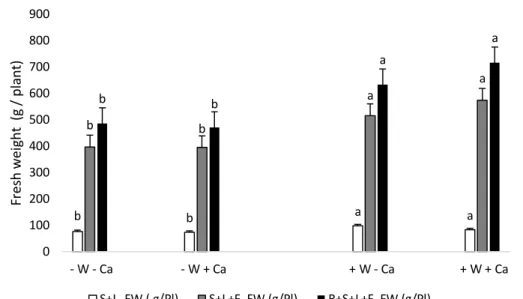
(13) Biomass The fresh biomass of every plant was separated in shoots + leaves (S+L), shoots + leaves + fruits (S+L+F) and roots + shoots + leaves + fruits (R+S+L+F). Figure 4 shows the mean for each treatment, in all cases the water factor was responsible of the differences between treatments. Thus, -W-Ca and –W+Ca have the lowest values of fresh weight, in contrast with the higher weigh of the +W-Ca and +W+Ca treated plants.. 900 a. Fresh weight (g / plant). 800 a. 700. a. 600. b. 500. a. b. b. b. 400 300 200 100. b. a. b. a. 0 - W - Ca S+L FW ( g/Pl). - W + Ca S+L+F FW (g/Pl). + W - Ca. + W + Ca. R+S+L+F FW (g/Pl). Figure 4. Fresh weight of shoots+leaves (S+L), shoots+leaves+fruits (S+L+F) and roots+shoots+leaves+fruits (R+S+L+F) of water stress and without calcium supply plants (-W-Ca); water stress and calcium supply (-W+Ca); optimum watering and without calcium supply (+W-Ca); optimum watering and calcium supply (+W+Ca) treatments of tomato plants growing in pot. Means with different letters indicate significant differences (Tukey test, p < 0.05).. 13.
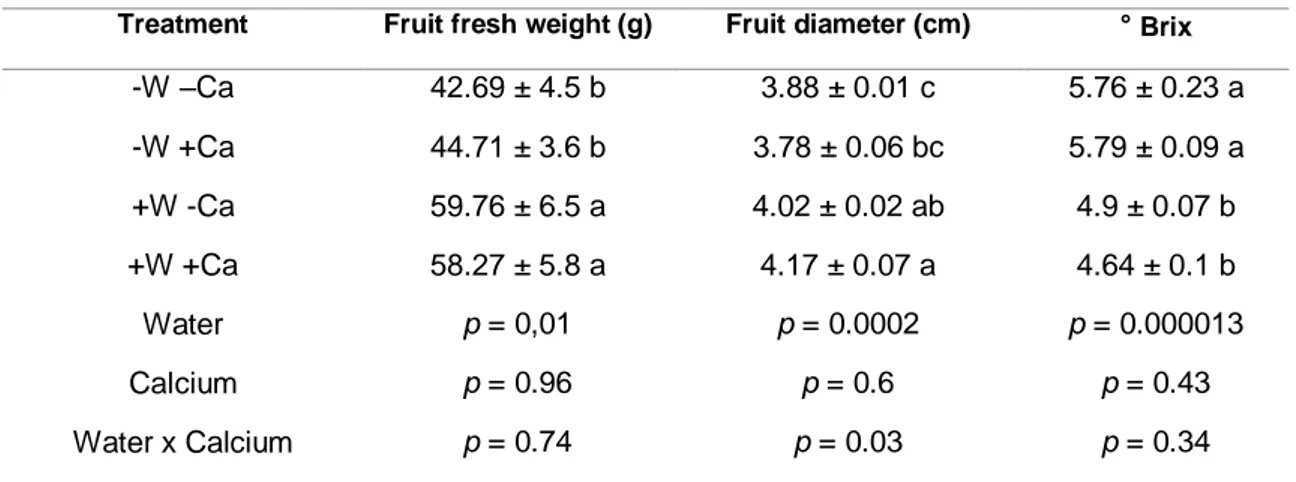
(14) Fruit parameters. Fruit weight, fruit diameter and soluble solids for each treatment are presented in Table 2, and their dry-weight concentration of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg) in Table 3. Data on the standard germination of seeds from the different treatments is displayed in Fig. 5. Table 2. Fresh weight, diameter and soluble solids in tomato fruits from of water stress and without calcium supply (-W-Ca); water stress and calcium supply (W+Ca); optimum watering and without calcium supply (+W-Ca); optimum watering and calcium supply (+W+Ca) treatments of tomato plants growing in pot. For each parameter, analysis of variance (ANOVA), results for each factor and their interaction is presented. Means with different letters indicate significant differences (Tukey test, p < 0.05). Treatment. Fruit fresh weight (g). Fruit diameter (cm). ° Brix. -W –Ca. 42.69 ± 4.5 b. 3.88 ± 0.01 c. 5.76 ± 0.23 a. -W +Ca. 44.71 ± 3.6 b. 3.78 ± 0.06 bc. 5.79 ± 0.09 a. +W -Ca. 59.76 ± 6.5 a. 4.02 ± 0.02 ab. 4.9 ± 0.07 b. +W +Ca. 58.27 ± 5.8 a. 4.17 ± 0.07 a. 4.64 ± 0.1 b. Water. p = 0,01. p = 0.0002. p = 0.000013. Calcium. p = 0.96. p = 0.6. p = 0.43. Water x Calcium. p = 0.74. p = 0.03. p = 0.34. 14.
(15) Table 3. Mineral composition (g/fruit) of tomato fruits of water stress and without calcium supply (-W-Ca); water stress and calcium supply (-W+Ca); optimum watering and without calcium supply (+W-Ca); optimum watering and calcium supply (+W+Ca) treatments of tomato plants growing in pot. For each parameter, analysis of variance (ANOVA) result (p value) for each factor and their interaction is presented. Means with different letters indicate significant differences in the same column (Tukey test, p < 0.05) (mean ± SEM). Treatment. Fruit N. Fruit P content. content -W –Ca. 0.28 ± 0.008 c. 0.08 ± 0.007bc. Fruit K. Fruit Ca. Fruit Mg. content. content. content. 0.44 ± 0.046 c. 0.020 ± 0.001 b. 0.025 ± 0.002 b. -W +Ca. 0.30 ± 0.01 bc. 0.07 ± 0.004 c. 0.46 ± 0.029 c. 0.018 ± 0.002 b. 0.024 ± 0.001 b. +W -Ca. 0.36 ± 0.007 b. 0.10 ± 0.005 b. 0.61 ± 0.035 b. 0.031 ± 0.002 a. 0.033 ± 0.001 a. +W +Ca. 0.50 ± 0.04 a. 0.12 ± 0.007 a. 0.74 ± 0.035 a. 0.026 ± 0.002 a. 0.039 ± 0.002 a. Water. p = 0.00008. p = 0.0002. p = 0.00006. p = 0.002. p = 0.00007. Calcium. p = 0.005. p = 0.23. p = 0.06. p = 0.22. p = 0.22. Water x. p = 0.02. p = 0.06. p = 0.16. p = 0.47. p = 0.15. Calcium. 100. Standard germination (%). 90 80 70 60 50 40 30 20 10 0 - W - Ca. - W + Ca. + W - Ca. + W + Ca. Figure 5. Seed standard germination of seeds from of water stress and without calcium supply (-W-Ca); water stress and calcium supply (-W+Ca); optimum watering and without calcium supply (+W-Ca); optimum watering and calcium supply (+W+Ca) treatments of tomato plants grown in pots.. 15.
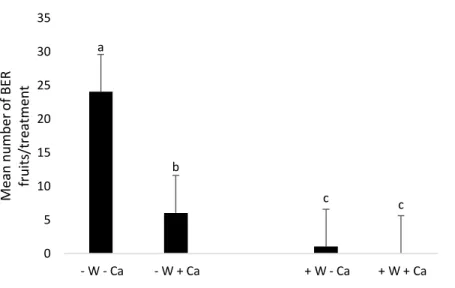
(16) Blossom end rot development. The proportion of fruits affected by BER as a result of water and calcium treatments was also evaluated (Figure 5). Water-stressed tomatoes without calcium supply had the highest percentage of BER-affected fruits (16.9%), while -W+Ca had six fruits with disorder (3.9%), +W-Ca: 1 (0.57%) and +W+Ca: 0 (0%).. 35. Mean number of BER fruits/treatment. 30. a. 25 20 15. b 10. c. c. + W - Ca. + W + Ca. 5 0 - W - Ca. - W + Ca. Figure 6. Mean number of tomato fruits with BER disorder as influenced by water stress and no calcium supply (-W-Ca); water stress and calcium supply (-W+Ca); optimum watering and without calcium supply (+W-Ca); optimum watering and calcium supply (+W+Ca) treatments of tomato plants grown in pots. Means with different letters indicate significant differences (Tukey test, p < 0.05).. The fruits with blossom end rot showed an early maturation in contrast with the other healthy fruits in the same cluster (Fig.7d, e). Sections of healthy fruits and with blossom end rot were observed in the electron scanning microscope, the section shown in Figure 8 is specifically from the area where visible damage can be seen.. 16.
(17) Figure 7. Images a, b and c show the development of fruits with blossom end rot from the stage of flowering (a) to mature fruit (c). Images d and e show the difference in maturation between healthy fruits and fruits with blossom end rot in the same cluster. In e, different grades of blossom end rot in tomato fruit at harvest.. 17.
(18) Figure 8. Scanning electron microscopy images of healthy tomato fruit (control) and fruit with blossom end rot (BER); cuts were made in the distal part of the fruit.. Calcium fractions. Calcium fractions were measured in healthy and BER-affected tomato fruits, following the methodology of Bonomelli et al., 2018. Fig. 6 shows a schematic representation of the average (n=6) amount of calcium that normal and BERaffected tomato fruits presented, along with the partitioning of calcium pectates, oxalates and silicates.. Figure 6. Diagram of total calcium distribution in healthy tomato fruits and fruits affected by blossom end rot, with average partitioning of calcium compounds (calcium pectates, oxalates and silicates).. 18.
(19) Discussion According to the observed results, during tomato fruit set existed significant differences on stem water potentials among treatments, were irrigated tomato plants (+W) clearly showed higher potential values, thus better water status compared with non-irrigated plants (-W). This result is in agreement with the consideration that plant water status is dependent on the water availability to the root system and the atmospheric water demand (Da Silva et al., 2018). According to Takayama y Nishima (2007), tomato plants reach critical water stress levels when having less than -1.5 MPa. However, Torrecillas et al. (1995) reported that non-irrigated tomato plants that reached -0.7 MPa of leaf water potential, had their stomatal conductance (gs) was greatly reduced when compared with watered plants. In the present work, tomato plants with no irrigation during fruit set reached -1 MPa, indicating a considerable water stress.. Plant water deficits result in low gs and Pn rates. Osmotic adjustment helps to maintain pressure potential in the cells and this allows leaf extension and photosynthesis to continue under stress (Morgan, 1984). Our results showed that Pn and gs were lower when less water was applied.. With regard to the histological analysis, the images show that the pedicel xylem tissue in tomato treated with no irrigation and no calcium application (-W-Ca) (whose plants presented most of BER-affected fruits in this work) showed predominance of protoxylem traqueary elements, and a less defined and/or less developed xylem tissue compared to plants in treatment +W+Ca. This could indicate that the lack of calcium combined with less movement of sap in the xylem, derived from a water deficit, can affect the formation of xylem tissue during the development of the fruit. Calcium is fundamental for the development of cell wall in all tissues, including xylem (Wyn Jones and Lunt, 1967), since it is a component of the middle lamela (Wyn Jones and Lunt, 1967). In addition, Ca2+ has another important role in xylem tissue formation, since it modifies the speed of auxin transport (De la Fuente, 1984); xylem tissue development has been associated with auxin accumulation (Benkova et al., 2003).. A lack of xylem development can also reduce the conductivity of xylem sap hence affecting the development of the fruit, influencing its water content, growth and. 19.
(20) arrival of elements such as calcium, which moves mainly by xylem flow in an acropetal direction. The susceptibility of tomato cultivars to BER has been related to the differential xylem-borne Ca to the distal end of the fruit in response to the growing environment. In fact, fruit from cultivars susceptible to BER generally have lower Ca concentrations than fruits from non-susceptible cultivars, especially right in post-anthesis (Franco et al., 1994; Willumsen et al., 1996). This may be a consequence of the reduced xylem network in fruit of cultivars susceptible to BER (Belda et al., 1996).. According to this, BER could be a primary consequence of an anatomical problem rather than a cellular signal triggered by the lack of Ca perceived at the genetic level as raised by Ho and White (2005).. Total biomass and biomass partitioning were also affected significantly by water stress. Not all stages of development are equally sensitive to soil moisture deficits; the flowering and fruit setting stages of tomato have long been known to be the most sensitive to water deficits in terms of yield (Salter, 1954). Lower or deficitary irrigation rates generally decrease yield and fruit size (Giardini et al., 1988). In our case the yield, fresh weight of shoots, leaves, fruits and roots decreased in plants with –W treatments. Growth inhibition is followed by less carbon assimilation, imbalanced mineral nutrition and accumulation of abscisic acid (ABA), which cause wilting of plants (Farooq et al., 2012; Lisar et al., 2012). The negative effects of drought stress on mineral nutrition and metabolism result in reduction of leaf area and disruption of assimilate partitioning. Considerable reduction in leaf area and associated morphological and physiological traits in sunflower lines under drought stress (Fernández-Moroni et al., 2012; Lisar et al., 2012; Hussain et al. 2018).. Fruit weight and diameter were lower in water stress treatments. Birhanu and Tilahun (2010) reported a decreased number and sizes of tomato fruits from plants subjected to moisture stress. The same observation of water stress on tomato yield parameters was also reported by Zotarelli et al., (2009). Sibomana et al., (2013) reported that general growth and yield of tomato plants subjected to severe water stress were significantly reduced compared to the well-watered plants. On the other hand, soluble solids were more concentrated in fruits from plants under water stress (Table 2). May (1993) reported that water stress did not result in low soluble solids, while high stress level resulted in the highest soluble solids and the poorest. 20.
(21) viscosity Similar results were found by Yrisarry et al. (1993), Branthome et al. (1994), Hanson et al. (1997), Colla et al. (1999), and Candido et al. (2000). Patane and Cosentino (2010) observed that the greatest effect of increasing soil water deficit was the rise in soluble solids (Ozbahce and Tari, 2010).. The results of mineral composition of fruits showed that the differences in N, P, K, Ca and Mg between treatments were driven mostly by the water factor, with Ca treatment influencing only N and K concentration. In the case of N, we found an interaction WxCa, that is, both water and calcium are affecting its content in the fruit.. Water stress also affects plant mineral nutrition and disrupts ion homeostasis. Calcium plays an essential role in structural and functional integrity of plant membrane and other structures. A decrease in plant Ca2+ content under water stress has been reported in many plants; for example, a decrease of about 50% in Ca2+ happened in drought stressed maize leaves, while root Ca 2+ concentration was higher than that of a well-watered control. Potassium is an important nutrient and plays an essential role in water relations, osmotic adjustment, stomatal closure and finally plant resistance to drought. Decrease in K + concentration was reported in many plant species under water deficient condition, mainly due to membrane damage and disruption in ion homeostasis. K + deficient plants have lower resistance to water stress. Nitrogen metabolism is the most important factor that influences plant growth and performance. Disruption in N metabolism is a crucial in-plant injury under the water deficit conditions. Some studies showed a reduction of nitrate uptake and decrease in nitrate reductase activity under water stress (Lisar et al., 2012).. The results of seed germination analysis showed that the standard germination, or potential of a seed lot to produce normal seedlings, was not affected by the water or calcium supply to the fruit-bearing plants. This would be associated with the ability of the stressed plant to adjust it growth by the relationship between vegetative sources and reproductive sinks (Wery, 2005). In this study, stressed plants produced less fruits and seeds, however individual seed quality was not affected. Similar results has been observed in alfalfa (Shock et al., 2007), wild bushbean (Santos et al., 2006), and lettuce (Contreras et al. 2008) when comparing seeds from plants grown with different water availability; however, none study regarding the effect of maternal calcium deficit on produced seed quality was found.. 21.
(22) Calcium fractions were measured in three different segments of the fruit (basal, medium and distal) following the methodology of Bonomelli et al. 2018. Which was measured independently in every third portion of the fruit to know the gradient of concentration and distribution in both healthy and BER-affected fruits. The results show that there is a higher concentration of calcium in the part near the peduncle in both cases, and that it decreases as we get closer to the distal part of the fruit (Figure 6). In the fruit, Ca2+ concentration decreases from the peduncle towards to the distal end tissue (Lewis and Martin 1973; Nonami et al. 1995; De Freitas and Mitcham; 2012). Accordingly, distal fruit tissue is more susceptible to Ca2+ deficiency disorders than fruit tissue at the peduncle region and Ca 2+ deficiency symptoms usually begin in the distal tissue, eventually spreading to the whole fruit in severe cases (White and Broadley 2003; Ho and White 2005). The reason for such fruit Ca2+ distribution is not well understood, but different mechanisms can potentially be involved such as cell wall Ca 2+-binding capacity and symplastic Ca 2+ uptake in the peduncle end tissue, abundance of functional xylem vessels from peduncle to distal fruit tissue, as well as the driving force required for Ca 2+ translocation from peduncle to distal fruit tissue (hydrostatic gradient) (De Freitas and Mitcham; 2012). The results obtained with respect to the partition of oxalates, silicates and calcium pectates show that the healthy fruit has more calcium pectates than the fruit with BER. This exchangeable calcium is responsible for cell wall stability, hence the loss of cellular structure in BER-affected tomato fruits (Saure, 2005). As for the more insoluble forms of calcium such as oxalates and silicates, our results show a greater proportion of these compounds in fruits with BER than in healthy fruits; these insoluble forms are considered inactive forms of calcium (Saure, 2014).. With our research we sought to identify the relative importance of water and calcium supply on the induction of BER. Most of the fruits that presented this physiological disorder were present in the treatments with water stress, (-W-Ca), followed by -W + Ca with a 75% reduction on number of affected fruits. On the other hand, in the case of the well-watered treatments, +W-Ca presented only one fruit with blossom end rot, while in the control treatment (+W+Ca) none BER damaged fruit was found. According to these results, although the water factor is the main responsible for the development of fruits with BER, the combined -W-Ca treatment was the most unfavorable condition for the plant.. 22.
(23) The fruits with BER presented an early maturation in comparison with the healthy fruits of the same cluster (Figure 7d, e). Nutrient deficit and abiotic stresses will both induce a strong hormonal response in the plant in order to adapt to the new restricted conditions. The changes include an increase in ABA and a reduction in auxin among others (Shinozaki and Yamaguchi-Shinozaki, 2000; Tuteja, 2007; Raghavendra et al., 2010; Ye et al., 2012). It has been proposed that exogenous GA treatments and GA mutant lines can induce the development of BER in tomato (De Freitas et al., 2011). However, it is still unclear whether endogenous GA is changing in fruits developing BER. The changes in fruit development induced by BER in our work are in agreement with unaltered balance in the fruit hormonal crosstalk that leads to maturation and ripening.. By observing the cuts made in the area of the fruit that presents the visible damage compared to a cut in the same area but of a healthy fruit in the electron scanning microscope, different cell structures were observed. BER fruit shows a complete deterioration of the cell structure, opposite that que well-structured and compartmentalized cells in healthy fruit.. Conclusions. The results of biomass, yield, fruit quality and nutrient content changed with the water factor (-W treatments) only the seed germination analysis showed that the standard germination, or potential of a seed lot to produce normal seedlings, was not affected by the water or calcium supply to the fruit-bearing plants, stressed plants produced less fruits and seeds, however individual seed quality was not affected.. In conclusion according to these results, although the water factor is the main responsible for the development of fruits with BER, the combined -W-Ca treatment was the most unfavorable condition for the plant. This treatment (-W-Ca) also showed predominance of protoxylem traqueary elements, and a less defined and/or less developed xylem tissue compared to plants in treatment +W+Ca. This could indicate that the lack of calcium combined with less movement of sap in the xylem, derived from a water deficit, can affect the formation of xylem tissue during the development of the fruit. This difficult the movement of calcium towards the fruit by. 23.
(24) the xylem and according to this, BER could be a primary consequence of an anatomical problem. The distribution of calcium within the fruit was affected in the case of the fruits that presented BER, healthy fruits have more soluble calcium than fruits that present this physiological disorder. Farther investigations are necessary to understand the cause of BER in tomatoes and the effects they have on the hormonal signaling that is triggering an early maturation.. References Belda RM, Fenlon JS, Ho LC. 1996. Salinity effects on the xylem vessels in tomato fruit among cultivars with different susceptibilities to blossom-end rot. Journal of Horticultural Science 71: 173–179. Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. Birhanu, K. and Tilahun, K. 2010. Fruit yield and quality of drip-irrigated tomato under deficit irrigation. African Journal of Food Agriculture Nutrition and Development. 10(2), 2139-2151. Bonomelli C., Arias M.I. and Villalobos L. 2018. Adaptation and Validation of a Methodology for the Measurement of Calcium Fractions in Fruits. Communications in Soil Science and Plant Analysis. Bonomelli, C. and Ruiz, R. 2010. Effects of foliar and soil calcium application on yield and quality of table grape cv. ‘thompson seedless’. Journal of Plant Nutrition, 33:299–314. Branthome, X.Y., Ple, J., Machado, R., Bieche, B.J., 1994. Influence of drip irrigation on the technological characteristics of processing tomatoes. Fifth International Symposium on the Processing Tomato, 23–27, Sorrento, Italy. Acta Horticulturae 376, 285–290. Candido, V., Miccolis, V., Perniola, M., 2000. Effects of irrigation regime on yield and quality of processing tomato (Lycopersicon esculentum Mill.) cultivars. III International. Symposium. on. Irrigation. of. Horticultural. Crops.. Acta. Horticulturae (ISHS) 537, 779–788. Colla, G., Casa, R., Cascio, B., Saccardo, F., Temperini, O., Leoni, C., 1999. Responses of processing tomato to water regime and fertilization in Central Italy. Acta Horticulturae 487, 531–535.. 24.
(25) Contreras, S., M.A. Bennett, and D. Tay. 2008. Restricted water availability during lettuce seed production decreases seed yield per plant but increases seed size and water productivity. HortScience, 43(3): 837-844. Cornell, 2015. Blossom End Rot. Cornell University: Cornell University’s Plant Disease Diagnostic Clinic of Department of Plant Pathology and Plantmicrobe Biology. Da Silva, C., Da Silva CA, De Freitas, C., Golynski, A., Da Silva, FM., Frizzone, JA. 2018. Tomato water stress index as a function of irrigation depths. Rev. bras. eng. agríc. ambient. 22: 95-100. De Freitas ST, Shackel KA, Mitcham EJ. 2011. Abscisic acid triggers whole-plant and fruit-specific mechanisms to increase fruit calcium uptake and prevent blossom end rot development in tomato fruit. Journal of Experimental Botany 62, 2645–2656. De Freitas, S.T, and E. J. Mitcham. 2012. Factors involved in fruit calcium deficiency disorders. In: Horticultural Reviews, ed. J. Janick, vol. 40, pp. 107–146. New York: Wiley-Blackwell. De la Fuente R.K. 1984. Role Of calcium in the polar secretion of indoleacetic acid. Plant Physiol. 76: 342–346. Dong C., Zhou J., Duan Z., Fan X., Wang H. 2001. Studies on the mechanism of tomato blossom end rot. J. Hortic. (28 Suppl), pp. 644-648. Farooq, M., Hussain, M., Wahid, A., Siddique, K.H.M., 2012. Drought stress in plants: an overview. In: Aroca, R. (Ed.), Plant Responses to Drought Stress: From Morphological to Molecular Features. Springer-Verlag, Germany, pp. 1–36. Ferguson, I.B., Watkins, C.B., 1989. Bitter-pit in apple fruit. Hortic. Rev. 11, 289– 355. Fernández-Moroni, I., Fraysse, M., Presotto, A., Cantamutto, M., 2012. Evaluation of Argentine wild sunflower biotypes for drought stress during reproductive stage. Helia 35 (57), 29–36. Franco JA, Bañón S, Madrid R. 1994. Effects of a protein hydrolysate applied by fertigation on the effectiveness of calcium as a corrector of blossom-end rot in tomato cultivated under saline conditions. Scientiae Horticulturae 57: 283– 292. Giardini, L., R. Giovanardi, and M., Borin. 1988. Water consumption and yield response of tomato in relation to water availability at different soil depth. Acta Hort. 228:119-126.. 25.
(26) Hanson, B.R., May, D.M., Schwankl, L.J., 1997. Drip irrigation of processing tomatoes. In: ASAE Annual International Meeting, Minneapolis, Minnesota, USA, 10–14 August 1997. Ho LC, White PJ. 2005. A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Annals of Botany 95, 571–581. Hoagland D.R., Arnon D.I. 1950. The water culture method for growing plants without soil. Calif Agric Exp Stn Circ, 347, pp. 1-38. Hocking B., Tyerman S.D., Burton R.A., and Gilliham M. 2016. Fruit Calcium: Transport and physiology. Front Plant Sci. 2016 Apr 29; 7:569. Hussain M., Farooq S., Hasan W., Ul-Allah S., Tanveer M., Farooq M. and Nawaz A. 2018. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agricultural Water Management, vol. 201, issue C, 152-166. International Seed Testing Association (ISTA). 2011. International rules for seed testing.. The. International. Seed. Testing. Association,. Basserdsdorf,. Switzerland. Kalcsits, L.A. 2016. Non-destructive Measurement of Calcium and Potassium in Apple and Pear Using Handheld X-ray Fluorescence. Frontiers in Plant Science. Vol 7. 442. Leuning R., Sands P. 1989. Theory and practice of a portable photosynthesis instrument. Plant Cell Environ., 12, pp. 669-678. Lewis, T.L. and D. Martin. 1973. Longitudinal distribution of applied calcium, and of naturally occurring calcium, magnesium, and potassium, in Merton apple fruits. Austral. J. Agric. Res. 24:363–371. Lisar, S.Y., Rahman, I.M., Hossain, M.M., Motafakkerazad, R., 2012. Water Stress in Plants: Causes, Effects and Responses. INTECH Open Access Publisher. Lyon C.B., Beeson K.C., Barrentine M. 1942. Macro-element nutrition of the tomato plant as correlated with fruitfulness and occurrence of blossom-end rot. Bot. Gaz., 103, pp. 651-667 May, D.M., 1993. Moisture stress to maximize processing tomato yield and fruit quality. In: International Symposium on Irrigation of Horticultural Crops. Mead, R., Curnow, R.N., Hasted, A.M., 1993. Statistical Methods in Agriculture and Experimental Biology, 2nd Edition. Chapman & Hall/CRC Press LLC, New Yorkand Boca Raton, FL. Mi, S., 2012. Studies on Main Aroma Components and Nutritional Quality of Tomato Fruit. Yangzhou University, Yangzhou, pp. 3–24.. 26.
(27) Morgan, J. M. 1984. Osmoregulation and water stress in higher plants. Annu. Rev. Plant Physiology. 35:299-319. Nonami, H., T. Fukuyama, M. Yamamoto, L. Yang, and Y. Hashimoto. 1995. Blossom-end rot of tomato plants may not be directly caused by calcium deficiency. Acta Hort. 396:107–114. Ozbahce, A.; Tari, A.F. 2010. Effects of different emitter space and water stress on yield and quality of processing tomato under semi-arid climate conditions. Agric. Water Manag. 97, 1405–1410. Patane, C., Cosentino, S.L., 2010. Effects of soil water deficit on yield and quality of processing tomato under a Mediterranean climate. Agricultural Water Management 97, 131–138. Raghavendra, S., V.K. Gonugunta, A. Christmann, and E. Grill. 2010. ABA perception and signaling. Trends in Plant Science 15:395-401. Raleigh S.M., Chucka J.A. 1944. Effect of nutrient ratio and concentration on growth and composition of tomato plants and on the occurrence of blossom-end rot of the fruit. Plant Physiol., 19, pp. 671-678. Salter, PJ. 1954. The effects of water regimes on the growth of plants under glass. I. Experiments with tomatoes (lycopersicon esculentum Mill.). J. Hort. Sci. 29:258-268. Santos, A.M.D., L.M.G. Rosa, L.B. Franke, and C. Nabinger. 2006. Heliotropism and water availability effects on flowering dynamics and seed production in Macroptilium lathyroides. Rev. Bras. Sementes 28:45-52. Saure, M.C. 2001. Blossom-end rot of tomato (Lycopersicon esculentum Mill.): A calcium or a stress-related disorder? Scientia Hort. 90:193–208. Saure, M.C. 2005. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Scientia Hort. 105:65–89. Saure, M.C. 2014. Why calcium deficiency is not the cause of blossom-end rot in tomato and pepper fruit a reappraisal. Scientia Horticulturae Vol. 174: 151154. Scholander PF, Hammel HT, Hemmingsen EA, Bradstreet ED. 1964. Hydrostatic pressure and osmotic potential in leaves of mangroves and some other plants. Proceedings of the National Academy of Sciences, USA 52,119–125. Shao T., Zhao J., Zhu T., Chen M., Wu Y., Long X., Gao X. 2018. Relationship between rhizosphere soil properties and blossom-end rot of tomatoes in coastal saline-alkali land. Applied Soil Ecology 127. 96–101. 27.
(28) Shear C.B. 1975. Calcium-related disorders of fruits and vegetables. HortScience, 10, pp. 361-365. Shinozaki, K., and K. Yamaguchi-Shinozaki. 2000. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Current Opinion in Plant Biology 3(3):217-223 Shock, C.C., E.B.G. Feibert, L.D. Saunder, and J. Klauzer. 2007. Deficit irrigation for optimum alfalfa seed yield and quality. Agron. J. 99:992-998. Sibomana, I. C., Aguyoh J. N. and Opiyo A. M. 2013. Water stress affects growth and yield of container grown tomato (lycopersicon esculentum mill) plants. G.J.B.B., VOL.2 (4) 2013: 461-466. Soltanpour, P.N.; Jones, J.B., Jr.; Workman, S.M. 1982. Optical Emission Spectrometry. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A., Ed.; American Society of Agronomy: Madison, WI, USA. pp. 29–65. Suzuki, K., M. Shono and Y. Egawa. 2003. Localization of calcium in the pericarp cells of tomato fruits during the development of blossom-end rot. Protoplasma. 222: 149–156. Takayama K., Nishima, H. 2007. Early Detection of Water Stress in Tomato Plants Based on Projected Plant Area. Environmental control in biology 45:241-249. Taylor M.D. and Locascio J. 2004. Blossom-end rot: A calcium deficiency. J. Plant Nutr. 27: 123-139. Torrecillas, A., Guillaume, C., Alarcón, J., Ruiz-Sánchez, MC. 1995. Water relations of two tomato species under water stress and recovery. Plant Science 105: 169-176. Tuteja, N. 2007. Review abscisic acid and abiotic stress signaling. Plant Signaling & Behavior 2(3):135-138. Wery, J. 2005. Differential effects of soil water deficit on the basic plant functions and their significance to analyze crop responses to water deficit in indeterminate plants. Aust. J. Agr. Res. 56:1201–1209. White P.J. and Broadley M.R. 2003. Calcium in plants. Ann Bot 92:487–511. Willumsen, J., Petersen, K., Kaack, K. 1996. Yield and blossom-end rot of tomato as affected by salinity and cation activity ratios in the root zone. Journal of Horticultural Science and Biotechnology 71:81-98. Wyn Jones, R.G., Lunt, O.R. 1967. The function of calcium in plants. Bot. Rev. 33: 407–426.. 28.
(29) Ye, N., L. Jia, and J. Zhang. 2012. ABA signal in rice under stress conditions. Rice 5(1):1-9. Yrisarry, B.J.J., Losada, P.M.H., Podriguez, A.R., 1993. Response of processing tomato to three different levels of water and nitrogen applications. In: International Symposium on Irrigation of Horticultural Crops. Zhang H.Y. 2003. Protective effect of tomato and product development. Shanxi Food Ind., 3, pp. 17-19. Zotarelli, L., Scholberg, J.M., Dukes, M. D., Munoz- Carpena, R. and Icerman, J. 2009. Tomato yield, biomass accumulation, root distribution and irrigation water use efficiency on a sandy soil, as affected by nitrogen rate and irrigation scheduling. Agricultural water management, 9(6), 2 3–34.. 29.
(30)
Figure


Documento similar
Three irrigation treatments were applied: control treatment (CTL) irrigated to ensure non-limiting soil water conditions; moderate water stress (MS) subjected to two drying cycles
• Inadvertently, virtual water will play a greater role in food trade – simply as a function of increasing water scarcity and the disparity of water availability between countries
subjected to salt stress, there was a 3.9-fold increase of proline content in salt-stressed non-inoculated plants
midday leaf water potential (A) and midday stomatal conductance vs midday stem water potential (B) in response to different irrigation regimes in 3 year-old pear trees planted
Water potential ( Ψ w ) and relative water content (RWC) of the flag leaves in well-watered (WW) and water-stressed (WS) plants of durum wheat (Triticum turgidum L. durum) grown
Gebre and Kuhns [27] indicated that cottonwood plants submitted to water stress preconditioning using different irrigation intervals developed a limited osmotic adjustment of 0.2
attitudes with respect to the price they pay and would be willing to pay for irrigation water, as well as the marginal product value of the water they use and the potential for
Values of leaf osmotic potential at full turgor (Ψ os ) were significantly lower in stressed than in control plants at the end of each stress period, showing an osmotic