www.elsevier.es/rmuanl
REVIEW
ARTICLE
History
and
progress
of
antiviral
drugs:
From
acyclovir
to
direct-acting
antiviral
agents
(DAAs)
for
Hepatitis
C
O.L.
Bryan-Marrugo
a,
J.
Ramos-Jiménez
b,
H.
Barrera-Salda˜
na
a,
A.
Rojas-Martínez
a,
R.
Vidaltamayo
c,
A.M.
Rivas-Estilla
a,∗aDepartmentofBiochemistryandMolecularMedicine,SchoolofMedicine,‘‘Dr.JoséEleuterioGonzález’’,UniversityHospital,
UniversidadAutónomadeNuevoLeón,Monterrey,N.L.,Mexico
bDepartmentofInternalMedicine,SchoolofMedicine,‘‘Dr.JoséEleuterioGonzález’’,UniversityHospital,Universidad
AutónomadeNuevoLeón,Monterrey,N.L.,Mexico cUniversidaddeMonterrey,Monterrey,N.L.,Mexico
Received17December2014;accepted12May2015 Availableonline3July2015
KEYWORDS
HepatitisCvirus; Antiviraldrugs; Direct-acting antiviralagents (DAAs)
Abstract Thedevelopmentofantiviraldrugsisaverycomplexprocess.Currently,around50 drugs havebeenapprovedfor humanuseagainstvirusessuchasHSV,HIV-1,thecytomegalo virus,theinfluenzavirus,HBVandHCV.Advancementsinthisareahavebeenachievedthrough effortsandtechnicalbreakthroughsindifferentscientificfields.Theimprovementinthe treat-ment ofHCVinfectionisagoodexampleofwhatisneededforefficientantiviraltherapy.A thoroughdescriptionoftheeventsthatleadtothedevelopmentofspecificallytargeted antivi-raltherapyorHCV(STAT-C)couldbeusefultofurtherimproveresearchfortreatingmanyother viraldiseasesinthefuture.SimilartoHIV-1andHBVtreatment,combinationtherapyalongwith personalizedmedicineapproacheshavebeennecessarytosuccessfullytreatHCVpatients.This reviewisfocusedonwhathasbeendonetodevelopasuccessfulHCVtherapyandthedrawbacks alongtheway.
©2014UniversidadAutónomadeNuevoLeón.PublishedbyMassonDoymaMéxicoS.A.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/ by-nc-nd/4.0/).
Abbreviations: TFT,triflouro-thymidine;HCV,HepatitisCvirus;IFN,interferon;SVR,sustainedvirologicresponse;STAT-C,specifically
targetedantiviraltherapyforHepatitisC;DAAs,direct-actingantiviralagents.
∗Correspondingauthorat:DepartamentodeBioquímicayMedicinaMoleculardelaFacultaddeMedicinayHospitalUniversitario‘‘Dr. JoséEleuterioGonzález’’delaUniversidadAutónomadeNuevoLeón,Av.FranciscoI.MaderoyEduardoAguirrePeque˜nos/nCol.Mitras Centro,CP64460Monterrey,N.L.,Mexico.Tel.:+528183294174;fax:+528183337747.
E-mailaddress:amrivas1@yahoo.ca(A.M.Rivas-Estilla).
http://dx.doi.org/10.1016/j.rmu.2015.05.003
Introduction
From1972 to date, more than 50 newviruses have been identifiedasetiologicagentsofhumandisease.1Thesenew
viraldiseaseshaverequiredmoresophisticatedtherapeutic agents,butthedevelopmentprocessofthesestrategiesto thispointhasbeenslowandfullofhurdles.
Antiviralchemotherapyhasadvancedatsnail-likepace, unlikeantibiotics,whichin30yearsachievedanadvanced therapeuticstage.34yearselapsedfromthedescriptionof theantibacterialmoleculesalvarsan, ‘‘themagic bullet’’, byEhlrichin1910,2tothediscoveryofpenicillinbyFleming
in1929,3toDomagk’sdescriptionofprontosil,theprecursor
ofsulfonamidesin19354andtheisolationofstreptomycin,
chloramphenicol,erythromycin and tetracycline by Waks-manin1944.5However,ittookalmost60yearsforantiviral
developmentto reachits current status of effectiveness. The evolution of the treatment for Hepatitis C is a good exampleofhowcomplexantiviraldevelopmentcanbeand howacombinedandspecifictargetedantiviraltherapyhas provedtobethebestapproachtofollowfor viraldisease treatment.
TheHepatitisCvirus(HCV)affectsover170million indi-vidualsworldwide,80%ofwhicharechronicallyinfected.6
Thisisfourtimes thenumberofpeopleinfectedwithHIV and about half the number of persons infected with the HepatitisBvirus(HBV).7HCViscaused byahepatotropic
virus,whichbelongstotheFlaviviridaefamily,genus Hep-acivirus.HCVwasdiscoveredin1989anditsviralgenomeisa 9.6kb-longpositivesingle-strandedRNA.Itencodesasingle polyproteinprecursorof3010aminoacidsandhasan inter-nalribosomeentrysiteatthe5′ untranslatedregion.This
polyproteinprecursorisco-translationallyprocessedby cel-lularandviralproteasesintothreestructuralproteins(core, E1andE2)andsevennon-structuralproteins(p7,NS2,NS3, NS4A,NS4B,NS5AandNS5B).8Thestructuralproteins
asso-ciatewiththegenomicRNAandaviralparticleisassembled insidealipidicenvelope.
Treatment for HCV infection has come a long way. Between2001and2011,astandardofcare(SOC)forchronic HCV infection was established worldwide. It consisted of acombinationof pegylatedinterferon (PEG-IFN)and riba-virin(RBV). Nowadays, newspecific antiviral agents have beenapproved.InMay2011,boceprevirandtelaprevir,two first-generation NS3/4A protease inhibitors, were autho-rized for their use in combination withPEG-IFN and RBV for a24-to-48-week course oftreatment in HCV-genotype 1 infections. Two years later (December 2013), Simepre-vir (a second-generation NS3/4A protease inhibitor) was approved for use with PEG-IFN and RBV for a 12-week course of treatment in HCV-genotype 1, while sofosbu-vir(aNS5Bnucleotidepolymeraseinhibitor)wasapproved forusewithPEG-IFNand/or RBVfor a12/24-weekcourse of treatment in HCV-genotypes 1 to4. IFN-free regimens have been shown to give better results, because sofos-buvir, combined with simeprevir or an NS5A replication complexinhibitor (ledipasvirordaclatasvir),withor with-outRBV for a 12-week treatment in genotype 1, resulted inasustainedvirologicalresponse(SVR)>90%.Inaddition, ABT-450/r (ritonavir-boosted NS3/4A protease inhibitor)-based regimens, in combination with other direct-acting antiviral agent(s) with or without RBV for 12 weeks in
genotype 1, have demonstrated similar results regarding SVR.9
Roadblocks
for
antiviral
drug
development
As we see in the text above, therapy for HCV infection remainedalmostthesamefrom2001to2011.Afteradecade ofpoorlyeffectiveHCVtherapy,thedevelopmentofspecific compoundsagainstthisvirusrampedupHCVtreatmenton apacethatnearlymatched antiretroviraltherapyforHIV. ‘‘Whydidittakesuchalongtime?’’isanimportantquestion whose answer couldhelpontheapproaches towardsdrug developmentagainstuntreateddiseases.Thefirst complica-tionwhenstudyingavirusisthelimitationsregardinginvitro
systemsandanimalmodelsforexperimentation;second,is thelowrateofdiscoveryforefficientcandidatemolecules, and third,the delicatebalance betweenefficacy, toxicity and resistance towards the selected antiviral drug. Addi-tionaleconomicalaspectsmustalsobeconsidered.Herewe haveanalysedeachoftheseaspectsunderthelightofthe promises and pitfalls related toHepatitis C research and treatment.
HCV
study
tools
Virusesareintracellularorganismswhichdependoncellular machineryforreplication.Therefore,ahugebreakthrough in this field was achieved by Enders, Robbins and Weller in 1951, when they developed an in vitro virus propaga-tionsystemincellculture.10Sincethen,manyinvitroand
in vivo systems have been implemented for the study of severalviruses, suchaspolioandHIV. Cell assayssystems were recently developed for HCV infection and propaga-tion.IntheearlybeginningsofHCVstudies,nosmallanimal model existed tostudy HCVinfections, andChimpanzees, theonlyanimalscapableofbeinginfectedwithHCV,were precluded by both ethicaland functional difficulties.The
in vitro development for HCV research began with the sub-genomic replicon cell culture system that replicates autonomouslyinthehumanhepatomacelllineHuh-7 gener-atedbyBartenschlageretal.,in2001.11,12Thissub-genomic
repliconmodel wasfurtherimproved bytheidentification and introduction of adaptive mutations, which enhanced virusreplicationcapacityandleadtotheestablishmentof thefull-lengthrepliconsystem usingthehighlypermissive cell line Huh-7.5.1 in 2003, by Blight and Bartenschlager et al., separately.13---15 These developments allowed the
studyofHCVinfectionmechanisms,suchaspackaging, bud-ding andamoreaccurateevaluationof potentialantiviral molecules.Ontheotherhand,thedevelopmentofasmall animal model that can be infected with HCV became a reality with the T- and B-cell deficient mice with severe combined immunodeficiency (SCID), grafted with human hepatocytes. The firstHCVinfectionstudies inthis model were performed byMercer etal. in2001. Inrecent years thedevelopmentoftransgenicmicewithachimeric mouse-humanliverrevolutionizedHCVinfectionresearch,allowing theassessment ofpathologicaland immunologicalprofiles ofthedisease.16Today,scientistsrelyonacombination of
profilingin animalsas proxy indicators of antiviral drugs’ efficacy,beforeattemptingclinicaltrials.17
Screening
process
for
antiviral
drug
discovery
Anotheraspect that madeantiviral drugdiscovery a diffi-cultendeavorwasthelack ofastructuredandsystematic methodforantiviraldrugdevelopment.Threedecadesago most of the first discoveries of antiviral compounds were fortuitous, since molecules originally developed for other purposes were selected as antiviral candidates, based on their success in other medical disciplines. These meth-ods for antiviral discovery were empirical, and most of the time, the biological mechanism behind the observed antiviraleffectremainedunclear.Forinstance,theuseof thio-semicarbazonesagainstthevacciniavirus,describedin 1950byHamreetal.,andusedlaterasanantibacterialdrug against tuberculosis.18 In 1959, the 5-iodo-2-deoxyuridine
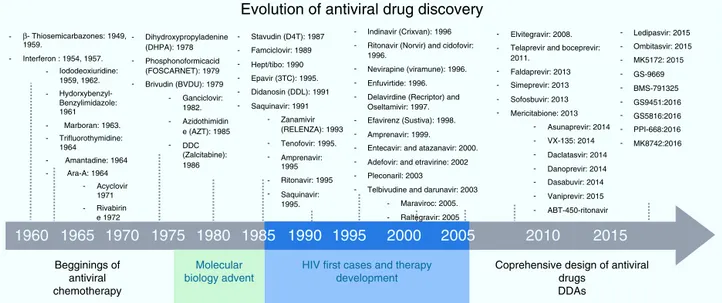
(IDU),whichwasoriginallydesignedforcancertreatment, provedtoexhibitantiviralactivityagainsttheHerpesVirus, butduetoitshighcytotoxicity,itsusewaslimitedtotopical application. IDUboostedantiviral development,and from its discovery many antiviral molecules were proposed for thetreatment ofvariousviraldiseases.19 InFig.1,atime
lineofthemilestonesinthedevelopmentofantiviralagents showstheearlyyearsofthisdisciplineandhowitevolvedto becomeastructuredandmethodicscience.20Atthetimeof
IDUdiscovery,onlyahandfulofviruseswereknowntocause diseasesinhumans.The firstantiviraldrugsweredirected totreatherpes,polio,smallpoxandinfluenza,astheywere themostrelevantviraldiseasesofthattime.Someofthem
thatwecanmentionarethefollowing:triflouro-thymidine (TFT), a nucleoside analogue used to treat herpes; ade-ninearabinoside(Ara-A)anucleosideanalogueagainstthe herpessimplexvirus21;2-(␣-hydroxybenzyl)benzimidazole
forthetreatmentofpoliomyelitis;Marboranforthe treat-mentofsmallpoxandamantadineandrimantadinetotreat influenza, which were identified by traditional biological screening assays in the early 1960s and was shown to be inhibitory for influenza A viruses in cell culture and animal models.In thelast twodecades,medicinal chem-istry hasdeveloped intoa recognizeddiscipline, in which a lead compound was usually identified by screening a large collectionof molecules. This method was improved withtheintroductionofcombinatorialchemistryand high-throughputscreening.22 Today,more structuredrationales
areimplementedwhenlookingfornewantiviraldrugs; sim-plescreening, blind screening and programmed screening have become more sophisticated, as the tools to ana-lyzestructure,proteininteractionandviralbehaviorhave evolved.ForHCVtherapydevelopment,manyattempts to treat the infection were implemented, with rather poor results.23 Duetothelack ofaserologicaltest, systematic
treatmentprotocolscouldnotbeperformed,andsoseveral ‘‘informal’’studieswerereported,evaluatingmanykindsof molecules.Butitwasnotuntil1986thatHoofnaglereported thebeneficialeffectofInterferonAlphainapilotstudyto treatNon-A/Non-Bhepatitis.24Thisreportprimedaboomin
HCVtherapeuticsandmanyrandomizedcontrolledclinical trialswereperformed toimproveHCVtreatment. In1990 RibavirinwasfirstproposedtotreatHCVinfectionandthe firstclinical trial for the assessment of itsefficacy began in 1991.25,26 After the efficacy of the combined antiviral
Receptor
Co-receptor Attachment
Virus
Replication
+ RNA Fusion
NS5A
Endoplasmic reticulus
Nucleus
Protease
Budding Maturation
Assembly
Protein maturation
RNA
dependent-RNA polymerase DNA
Uncoating
Ires directed translation
Poly-protein
Golgi membranes
None
None None
None
None
None
Boceprevir, telaprevir, sineprevir, faldaprevir, vaniprevir, MK5-172, GS-9451, danoprevir, asunaprevir.
Sofosbuvir, Meriditabine, VX-135, Dasabuvir, BMS-791325, GS-9669
Daclatasvir, ledipasvir, GS-5816, PPI-668, Ombitasvir, MK-8742
progenie Viral
Evolution of antiviral drug discovery
Dihydroxypropyladenine Stavudin (D4T): 1987 Indinavir (Crixvan): 1996 Elvitegravir: 2008. Ledipasvir: 2015 Ombitasvir: 2015 MK5172: 2015 GS-9669 GS9451:2016 GS5816:2016 PPI-668:2016 MK8742:2016 BMS-791325 2011. Faldaprevir: 2013 Simeprevir: 2013 Sofosbuvir: 2013 Mericitabione: 2013 Asunaprevir: 2014 VX-135: 2014 Daclatasvir: 2014 Danoprevir: 2014 Dasabuvir: 2014 Vaniprevir: 2015 ABT-450-ritonavir Telaprevir and boceprevir: Ritonavir (Norvir) and cidofovir:
1996.
Nevirapine (viramune): 1996.
Enfuvirtide: 1996.
Delavirdine (Recriptor) and Oseltamivir: 1997.
Efavirenz (Sustiva): 1998.
Amprenavir: 1999.
Entecavir: and atazanavir: 2000.
Pleconaril: 2003 Adefovir: and etravirine: 2002
Telbivudine and darunavir: 2003
Maraviroc: 2005. Famciclovir: 1989
Hept/tibo: 1990
Epavir (3TC): 1995.
Didanosin (DDL): 1991
Saquinavir: 1991 Zanamivir (RELENZA): 1993 Tenofovir: 1995. Amprenavir: 1995 1995. Ritonavir: 1995 Saquinavir: (DHPA): 1978 Phosphonoformicacid (FOSCARNET): 1979
Brivudin (BVDU): 1979
Ganciclovir: 1982.
Azidothimidin e (AZT): 1985
DDC (Zalcitabine): 1986 1959.
Interferon : 1954, 1957. Iododeoxiuridine: 1959, 1962. 1961 1964 Ara-A: 1964 Acyclovir
1960
Begginings of antiviral chemotherapy Molecular biology adventHIV first cases and therapy development
Coprehensive design of antiviral drugs
DDAs
1965
1970
1975
1980
1985
1990
1995
2000
2005
2010
2015
1971 Rivabirin e 1972 Amantadine: 1964 Marboran: 1963. Trifluorothymidine: Hydorxybenzyl-Benzylimidazole:
-β Thiosemicarbazones: 1949,
-- -Raltegravir: 2005
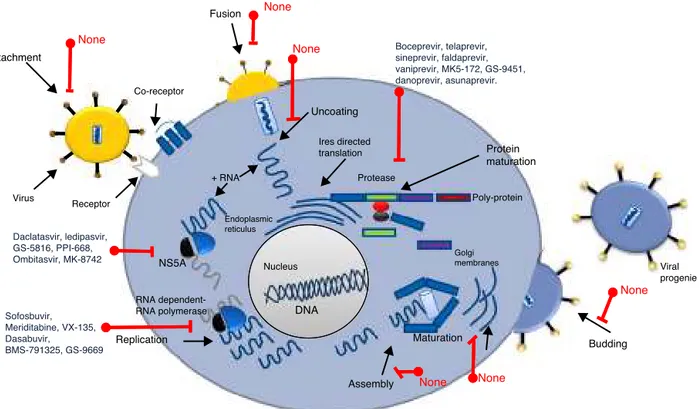
Figure2 HCVPotentialtargetsforantiviralchemotherapy.ManytargetsforantiviralactioncanbefoundalongHCV’slifecycle.
therapyofPegylatedInterferon-␣(PEG-IFN-␣)andribavirin againstHCVinfectionwasproven,itbecamethestandardof care(SOC)forthisdisease,anddespiteitsshortcomings(50% responserateand50%relapserateonpatientsinfectedwith genotype 1b, andunwanted side effects), it remained as suchformorethan15years.27,28Duringthistime,usingblind
screeningapproaches,somemoleculeswerefoundtoreduce HCV-RNAlevelsinvitro,butnoneofthemweresignificant enoughtobeimplementedclinically.ItwasnotuntilMay, 2011thattheimprovedunderstandingoftheHCVlifecycle ledtothediscovery,assessmentandFDAapprovaloftheHCV protease inhibitors Telaprevir and Boceprevir, that effec-tivelyreduceviral loadonchronicHCVinfected patients, in treatment of naïve patients and in prior relapsersand non-responders.29,30Telaprevirandboceprevirwerethefirst
direct-actingantiviralagents(DAAs)thatselectivelytarget HCV.However,newDDAshavebeenrecentlyaddedtothis list:simeprevir(proteaseinhibitor),sofosbuvir(NS5b poly-meraseinhibitor),daclatasvir(NS5Aproteininhibitor),and faldaprevir(second-waveNS3/4Aproteaseinhibitor),allof themshowing very promisingresults and some have even been proposedasthe treatment backbone for Interferon-freeHCVtherapies.31,32 WiththeseselectiveHCVprotease
inhibitors, the establishment of STAT-C therapy became a reality. Today, several DAAs (including HCV protease inhibitors,polymeraseinhibitors,andNS5Ainhibitors)arein variousstagesofclinicaldevelopment.Currentresearchis attemptingtoimprovethepharmacokineticsand tolerabil-ityoftheseagents,definethebestregimens,anddetermine treatmentstrategiesthatproducethebestoutcomes.Some of these DAAs will reach the market simultaneously, and resourceswillbeneededtoguidetheuseofthesedrugs.It isalsoworthmentioningthatdifferentlinesofresearchare currentlyevaluatingotherwaystoimproveHCV chemother-apy.Forexample,taribavirin,aprodrugforthelong-known nucleosideanalogue ribavirin, is at 3rdphase clinical tri-als and has shown promising results.33 This new antiviral
would further boost HCV therapy in the coming years.
Fig.2shows the majorHCVpotentialtargets for antiviral chemotherapy.
Efficacy
and
toxicity
on
the
development
of
an
efficient
antiviral
drug
SincethediscoveryofIDU50yearsago,onlyafewmolecules haveproventobeeffectiveandsafewhenusedfor selec-tive antiviral therapy. A huge breakthrough that came fromthebetterunderstandingofvirus-hostinteractionwas the inception of 9-(2-hydroxyethoxymethyl) guanine (Acy-clovir).Itwasthefirsthighlyselectiveantiviraldrug,beinga substratefortheHerpesSimplexVirus-encoded thymidine-kinase.It displayedadirectinhibitory effectagainstviral replicationandpracticallynoadverseeffectsonthehost. TheachievementofselectiveviraltoxicitybyAcyclovirand other similar molecules werethought of asthe beginning of anewtherapeuticagefor awell-established,effective and safe antiviral therapy.Acyclovir is a pro-drug, which meansithastobefurthermetabolizedinvivobefore enter-ing the infected cell wherein further metabolism may or maynotberequired toyield theactiveinhibitor.The key to Acyclovir’s specificity is the selective phosphorylation of the acyclic guanosine nucleoside by the Herpes virus-encoded pyrimidinedeoxynucleoside kinase, whichmeans it would only be active on Herpes-infected cells.34 After
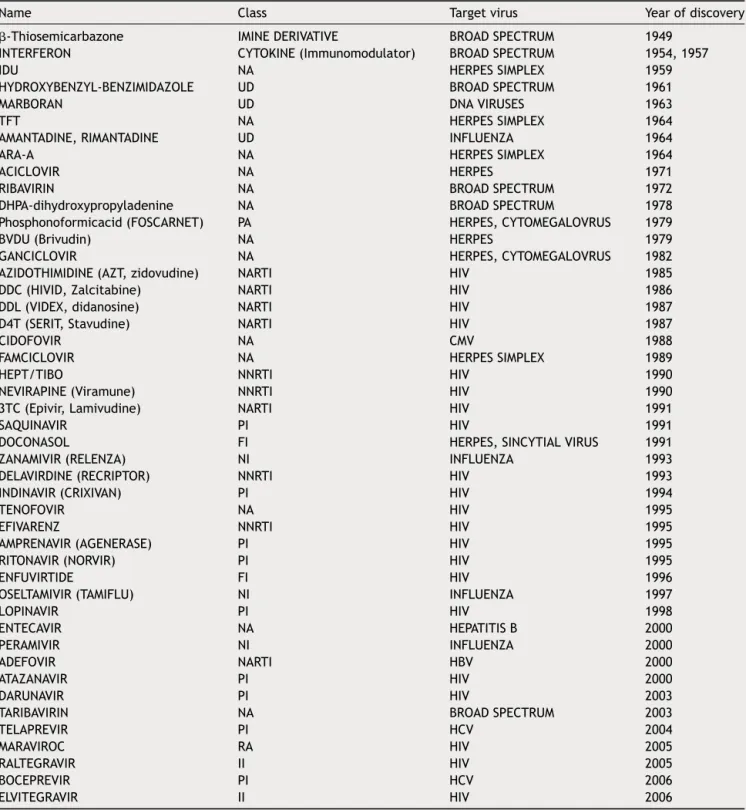
Table1 Majorantiviralcompoundsdevelopedandapprovedforuseinhumans.
Name Class Targetvirus Yearofdiscovery
-Thiosemicarbazone IMINEDERIVATIVE BROADSPECTRUM 1949
INTERFERON CYTOKINE(Immunomodulator) BROADSPECTRUM 1954,1957
IDU NA HERPESSIMPLEX 1959
HYDROXYBENZYL-BENZIMIDAZOLE UD BROADSPECTRUM 1961
MARBORAN UD DNAVIRUSES 1963
TFT NA HERPESSIMPLEX 1964
AMANTADINE,RIMANTADINE UD INFLUENZA 1964
ARA-A NA HERPESSIMPLEX 1964
ACICLOVIR NA HERPES 1971
RIBAVIRIN NA BROADSPECTRUM 1972
DHPA-dihydroxypropyladenine NA BROADSPECTRUM 1978
Phosphonoformicacid(FOSCARNET) PA HERPES,CYTOMEGALOVRUS 1979
BVDU(Brivudin) NA HERPES 1979
GANCICLOVIR NA HERPES,CYTOMEGALOVRUS 1982
AZIDOTHIMIDINE(AZT,zidovudine) NARTI HIV 1985
DDC(HIVID,Zalcitabine) NARTI HIV 1986
DDL(VIDEX,didanosine) NARTI HIV 1987
D4T(SERIT,Stavudine) NARTI HIV 1987
CIDOFOVIR NA CMV 1988
FAMCICLOVIR NA HERPESSIMPLEX 1989
HEPT/TIBO NNRTI HIV 1990
NEVIRAPINE(Viramune) NNRTI HIV 1990
3TC(Epivir,Lamivudine) NARTI HIV 1991
SAQUINAVIR PI HIV 1991
DOCONASOL FI HERPES,SINCYTIALVIRUS 1991
ZANAMIVIR(RELENZA) NI INFLUENZA 1993
DELAVIRDINE(RECRIPTOR) NNRTI HIV 1993
INDINAVIR(CRIXIVAN) PI HIV 1994
TENOFOVIR NA HIV 1995
EFIVARENZ NNRTI HIV 1995
AMPRENAVIR(AGENERASE) PI HIV 1995
RITONAVIR(NORVIR) PI HIV 1995
ENFUVIRTIDE FI HIV 1996
OSELTAMIVIR(TAMIFLU) NI INFLUENZA 1997
LOPINAVIR PI HIV 1998
ENTECAVIR NA HEPATITISB 2000
PERAMIVIR NI INFLUENZA 2000
ADEFOVIR NARTI HBV 2000
ATAZANAVIR PI HIV 2000
DARUNAVIR PI HIV 2003
TARIBAVIRIN NA BROADSPECTRUM 2003
TELAPREVIR PI HCV 2004
MARAVIROC RA HIV 2005
RALTEGRAVIR II HIV 2005
BOCEPREVIR PI HCV 2006
ELVITEGRAVIR II HIV 2006
NA:nucleosideanalogue;NARTI:nucleosideanalogue-reversetrancriptaseinhibitor;UD:undetermined;PA:pyrophosphateanalogue; NNRTI:non-nucleosideanalogue-reversetrancriptaseinhibitor;NARTI:nucleosideanalogue-reversetrancriptaseinhibitor;PI:protease inhibitor;FI:fusioninhibitor;NI:neuraminidaseinhibitor;RA:receptorantagonist;II:integraseinhibitor.
HIV.Thescienceofantiviralresearchwaswell established whenHIV/AIDS appeared asamajorviraldisease inearly 1980s.Anincreaseofantiviraltherapystudieswithnoequal tookplacewhenthefirstcasesofHIVwerereported. Azi-dothymidine(AZT),amongotherantiviralmoleculesalready inexistence,provedtohaveselectivetoxicityagainstHIV.
170
O.L.
Bryan-Marrugo
et
al.
Table2 SummaryoftherecenttreatmentguidelinesforHCVinfectiontherapy[describedbytheAmericanAssociationfortheStudyofLiverDiseases(AASLD),Infectious DiseaseSocietyofAmerica(IDSA)andtheInternationalAntiviralSociety(IASUSA)].
HCVgenotype Naïvepatientsb Non-responderstotraditionalIFN-RBV
therapy
Resistantto Sofosbuvir
Resistantto traditional therapyand1st generation protease inhibitors
Patientswith Cirrhosisb
1a CombinationofLedispavir90mg/sofosbuvir
400mgfor12wks
CombinationofLedispavir90mg/sofosbuvir 400mgfor12wks
Combinationof Ledispavir 90mg/sofosbuvir 400mgfor12 wks
Extend treatmentfor 24wks
Paritaprevir150mg/ritonavir
100mg/ombitasvir25mg/twicedailydose ofdasabuvir250mgandRBVafor12wks
Paritaprevir150mg/ritonavir
100mg/ombitasvir25mg/twicedailydose ofdasabuvir250mgandRBVafor12wks
CombinationOf Ledispavir 90mg/sofosbuvir 400mgplus RBVafor12
wks.
Extend treatmentfor 24wks
Sofosbuvir400mg/Simeprevir150mg/RBVa
for12wks
Sofosbuvir400mg/Simeprevir150mg/RBVa
for12wks
Extend treatmentfor 24wks
1b Ledipasvir90mg/sofosbuvir400mgfor12
wks
Ledipasvir90mg/sofosbuvir400mgfor12 wks
Ledispavir 90mg/sofosbuvir 400mgplus RBVa
CombinationOf Ledispavir 90mg/sofosbuvir 400mgfor12 wks
Extend treatmentto 24wks
Paritaprevir150mg/ritonavir
100mg/ombitasvir25mg/twicedailydose ofdasabuvir250mgfor12wks
Paritaprevir150mg/ritonavir
100mg/ombitasvir25mg/twicedailydose ofdasabuvir250mgfor12wks
CombinationOf Ledispavir 90mg/sofosbuvir 400mgplus RBVafor12
wks.
PlusRBV
Sofosbuvir400mg/Simeprevir150mgfor12 wks
Sofosbuvir400mg/Simeprevir150mgplus RBVafor12wks
Extend treatmentfor 24wks
2 Sofosbuvir400mgandRBVafor12wks Sofosbuvir400mgandRBVafor12wks Extend
treatmentfor 16wks Sofosbuvir400mgandRBVaplusweekly
PEG-IFNfor12wks
History
and
progress
of
antiviral
drugs
171
Table2 (Continued)
HCVgenotype Naïvepatientsb Non-responderstotraditionalIFN-RBV
therapy
Resistantto Sofosbuvir
Resistantto traditional therapyand1st generation protease inhibitors
Patientswith Cirrhosisb
Sofosbuvir400mg/RBVaplusWeekly
PEG-IFNfor12wks
Sofosbuvir400mgandRBVaplusweekly
PEG-IFNfor12wks
4 Ledipasvir90mg/Sofosbuvir400mgfor12
wks
Ledipasvir90mg/Sofosbuvir400mgfor12 wks
Paritaprevir150mg/ritonavir
100mg/ombitasvir25mg/andRBVafor12
wks
Paritaprevir150mg/ritonavir
100mg/ombitasvir25mg/andRBVafor12
wks
Sofosbuvir400mg/RBVafor24wks Sofosbuvir400mgplusRBVaplusWeekly
PEG-IFNfor12wks Sofosbuvir400mgplusRBVaplusWeekly
PEG-IFNfor12wks
Sofosbuvir400mgplusRBVafor24wks
5 Sofosbuvir400mgplusRBVaplusWeekly
PEG-IFNfor12wks
Sofosbuvir400mgplusRBVaplusWeekly
PEG-IFNfor12wks
WeeklyPEG-IFNplusRBVafor48wks WeeklyPEG-IFNplusRBVafor48wks
6 Ledispavir90mg/Sofosbuvir400mgfor12
wks
Ledispavir90mg/Sofosbuvir400mgfor12 wks
Sofosbuvir400mgplusRBVaplusweekly
PEG-IFNfor12wks
Sofosbuvir400mgplusRBVaplusweekly
PEG-IFNfor12wks
a RBV(Ribavirin)dosageisweightbased(1000mg[<75kg]and1200mg[>75kg]).
Allindicationsrefertodailydosesunlessisotherwiseclarifiedinthetext. b Definitionsfortreatmentcriteria.42,43
(Treatment)Naïvepatient:ApersonwhohasneverundergoneanyHCVtherapy.
---RapidVirologicResponse(RVR):ItisdefinedasanundetectableHCVRNAatweek4oftreatment.
---SustainedVirologicalResponse(SVR):ItisdefinedasundetectableHCVRNA12weeks(SVR12)or24weeks(SVR24)aftertreatmentcompletion.
---Non-response:ReferstoapatientwhodonotachieveundetectableHCVRNAduringthefirst24weeksoftreatment.Therearetwoformsofnon-responders:Partialrespondersand nullresponders.
---Partialresponse:Itisasub-categoryofnon-responseanddescribesadecreaseinHCVRNAlevelsbyatleast2Log10atweek12oftreatmentbutdetectablelevelsatweek24. ---Nullresponse:Isasub-categoryofnon-responseandreferstothesituationwhenapatientdoesnotsuppresstheirHCVRNAlevelsbyatleast2Log10byweek12oftreatment. ---Drugresistant:ApatientwhoisPartialorNullrespondertoaspecifictreatmentforwhichaHCV‘‘resistant’’mutantremainsimmunemakingnecessarytochangethetherapeutical approach.
onpublichealth.Althoughantiviralresearchand develop-ment were ignited by the HIV threat, many HIV patients werenotresponsivetothetreatment.ThediscoveryofAZT wasfollowedbyseveralotherdideoxynucleoside(ddN) ana-logues (ddI, ddC, d4T, 3TC, ABC, FTC) (Fig.2). All these NRTIsact ina similarfashion; aftertheir phosphorylation to triphosphates, they interact as ‘chain terminators’ of theHIV-reversetranscriptase,thuspreventingtheformation ofthe proviralDNA. Even though theyhad greatsuccess, drugresistance forced HIV treatment to evolve.Today, it isknownthat twoinevitable andimportantconsequences of antiviral therapy have to be taken into account when planning a treatment strategy for viral chronic diseases. Thefirstisthat,givenitsnature,long-termantiviral ther-apyautomaticallyselectsresistantmutantsthatwillsurvive andbecomedominantstrains.Resistant mutantsareeven morefrequentinviralthaninbacterialinfection,andthis becomesmoreevidentwhentreatingchronicviralinfections suchasHIVandHCV.35---37 Forviralinfections,anyattempt
toattackthevirus’metabolismcouldhaveaneffectonhost cells. It is evident then, that modifications of these two aspectsof antiviral therapy,could improve the results of treatmentforchronicpatients.This barrierwasovercome inpart throughtheuseof combinatorial therapy.In addi-tiontothat,theconceptofabroadspectrumoratleasta ‘‘pangenotypic’’antiviralmoleculethatcouldbeeffective on a wide range of viral pathogens is paradoxically self-defeatingifwethinkthatspecificityisrequiredtoavoidcell toxicityandtheoppositeisneededtobroadenthespectrum ofagivenantiviral molecule.Withourcurrent knowledge onviralmetabolismandhost interaction,threeaspectsof viralinfectioncanbetargetedforantiviraltreatment: inhi-bitionofviralgenesandproteins,blockingofhostgenesand enzymesthatinteractwithviralcounterparts,and modula-tionof host metabolic pathways involved inthe virus life cycle.
The
challenges
of
fighting
Hepatitis
C
As we mentioned before, a new era of therapeutics is currently emerging for Hepatitis C treatment, since sev-eral other direct-acting HCV antiviral drugs are being developed (Protease inhibitors: faldaprevir, asunaprevir, danoprevir,vaniprevir,ABT-450-ritonavir,MK5172,GS-9451; NS5A inhibitors: ledipasvir, ombitasvir, GS-5816, PPI-668, MK-8742 and daclatasvir; NS5b inhibitors: mericitabine, VX-135,dasabuvir,BMS-791325,GS-9669),whichhavebeen shown to reduce viral RNA levels, reaching SVR in up to 95%ofthetreatedpatients.38,39However,thereareseveral
challengestobeaddressedtocombatHCVusingnewdrugs. DAA’sdirectly attack the Hepatitis C virus and,similar to someofthedrugs usedtotreat HIV,thesenewmolecules targettheenzymesneededforviralproteinprocessing;the virusshouldcounterpartthiseffect(Fig.2).Basedonthat, HCVgeneticvariabilityand drugresistancearethebigger obstaclesthat DAAs must overcome. HCVhas a high rate ofreplication,with1012 virionsproduceddaily, alongwith
anequallyhighmutationrate,meaningthat,foranygiven drug,there are already resistant mutants present onthe infectedsubjectthatwouldultimatelyrendersingledrugs useless.However,HepatitisCresistancemaybedelayedor
prevented byusing combinationsofpotent antiviral drugs without cross-resistance profiles and optimizing patient adherence to therapy.38 On the other hand, accessibility
tothe new andapproved HCVtherapies is a challenge in combating the Hepatitis C, mainly because of the high cost of the combined treatments (between 100,000 and 250,000USD).Availabilityandaccessibilityofnewprotease inhibitors (PI), telaprevir, boceprevir, simeprevir, and the recentlyapprovedRNApolymeraseinhibitor(RPI)sofosbuvir dependsontheregionwherepatientsarelocatedandtheir accesstogovernmentalhealthprograms.Inmostcountries, accessibilitytothesedrugsispossibleonlyforthosepatients whocanaffordtreatmentforthemselves,aspublichealth systemsdonotyethavepoliciesforapplicationofthenew HCV therapy to the generalpopulation throughinsurance systems.40 This will likely require concerted public and
political mobilizationto pressure originator companiesto reduce prices andstimulategeneric competition.In addi-tion, lower prices could make widespread access to HCV treatmentpossibleinlowandmiddleincomecountries.
Where
we
stand
today
After almost 20 years since HCV’s discovery, today we account for a solid-yet-not-completely effective treat-ment landscapetofight hepatitisinfection. First,modern biomolecular diagnostictoolsareusedtodetermine geno-type and viral load as a base to design an accurate therapeutic regimen;second,viralloaddynamicsis moni-toredinordertodetermine drugresistance,andthirdthe liver’s state andthepresence ofinfectionareassessed in patients whohave completedthe therapy.In an effortto provideacondensedsetoftreatmentguidelines,the Amer-icanAssociationofLiverDisease(AASL),InfectiousDisease Society (IDSA)andthe InternationalAntiviralSociety (IAS-USA)generatedtheGuidelinesforHCVinfectiontreatment which are based on patient’s previous exposure to treat-ment,HCVgenotype,relapsingprofileandhepaticstatus.41
InTable2weshowacompendiumoftherecenttreatment guidelines for HCV infection therapy. It is important for physicianstoevaluatepatientclinicalhistory(naïveornot), HCVgenotype,treatmenteffectivenessandHIVco-infection inordertoavoidunwanteddruginteractions.
Conclusions
and
perspectives
virusfactorsarenottheonlypotentialtargetsforinhibition, buthosttargets areaswell,includingmicroRNAs,cellular receptors, adhesion molecules and cyclophilins. For the nearfuture,acombinationofhostandviralinhibitorswill provideavarietyofdrugregimesappropriatefordifferent patients that couldlead tointerferon-free therapies that canconsistentlycleartheinfection.
Aneweraof HCVtreatment andtheincreasing knowl-edge about viruses and their mechanisms of infection, combinedwiththerapiddiscoveryofnovelantiviral strate-giesandtechniques,willspeedupthedevelopmentofnovel antiviraldrugs.
Funding
FinancialsupportwasprovidedbytheCONACYT,grant num-berCB-2011-1-58781toA.M.R.E.
Conflict
of
interest
Theauthorshavenoconflictsofinterest.
Acknowledgements
WethankSergioLozano-Rodriguez,M.D.for hisassistance inreviewingthemanuscript.
References
1.DesselbergU.Emergingandre-emerginginfectiousdiseases.J Infectol.2000;40:3---15.
2.FitzgeraldJG.Ehrlich-HataremedyforSyphilis.CanMedAssoc J.1911;1:38---46.
3.PorritAE.Thediscoverydevelopmentofpenicillin.MedPress. 1951;19:460---2.
4.DomagkG.Sulfonamidesinthepastpresentandfuture.Minerva Med.1950;35:41---7.
5.NeuHC,GootzTD.Antimicrobialchemotherapy.Medical micro-biology. 4th ed. Galveston, TX: University of Texas Medical Branch;1996[Chapter11].
6.JangJY, ChungRT. New treatments for chronic hepatitis C. KoreanJHepatol.2010;16:263---77.
7.MoradpourD,PeninF,RiceCM.ReplicationofhepatitisCvirus. NatReviMicrobiol.2007;5:453---63.
8.BartenschlagerR,Ahlborn-Laake L, MousJ, et al.,Jacobsen H.Nonstructuralprotein3ofthehepatitisCvirusencodesa serine-typeproteaserequiredfor cleavageattheNS3/4and NS4/5junctions.JVirol.1993;7:3835---44.
9.FeeneyER,ChungRT.AntiviraltreatmentofhepatitisC.BrMed J.2014;349:3308.
10.RobbinsFC,EndersJF,WellerTH.Studiesonthecultivationof poliomyelitisvirusesintissueculture.V.Thedirectisolationand serologicidentification ofvirus strainsintissue culturefrom patientswithnon-paralyticand paralytic poliomyelitis.AmJ Hygene.1951;2:286---93.
11.BartenschlagerR,LohmannV.Novelcellculturesystemsforthe hepatitisCvirus.AntiviralRes.2001;1:1---17.
12.Lohmann V, Korner F, Kosch J, et al. Replication of subge-nomichepatitisCvirusRNAinahepatomacellline.Science. 1999;5424:110---3.
13.BlightKJ,McKeatingJA,MarcotrigianoJ,etal.Efficient repli-cationofhepatitisCvirusgenotype1aRNAsincellculture.J Virol.2003;5:3181---90.
14.WakitaT,PietschmannT,KatoT,etal.Productionofinfectious hepatitisCvirusintissueculturefrom acloneviralgenome. NatMed.2005;7:791---6.
15.BartenschlagerR,KaulA,SparacioS.Replicationofthe hepati-tisCvirusincellculture.AntivirRes.2003;2:91---102.
16.Ernst E, SchönigK, Bugert JJ. Generationof inducible hep-atitis C virus transgenic mouse lines. J Med Virol. 2007;8: 1103---12.
17.LinC.HCVNS3-4Aserineprotease.HepatitCVirusesGenomes MolBiol.2006;1:163---206.
18.Bauer D. Clinical experience with the antiviraldrug Marbo-ran(1-methyl.isatin3-thiosemicarbazone).AnnNYAcadSci. 1965;130:110---7.
19. Field HJ, De Clerk E. Antiviral drugs --- a short history of their discovery and development. Microbiol Today. 2004;31: 60---2.
20.Pierrel J.AnRNAphagelab:MS2 inWalterFiers’ laboratory ofmolecularbiologyinGhentfromgeneticcodetogeneand genome1963---1976.JHistBiol.2012;1:109---38.
21.Nesburn AB, Robinson C, Dickmson R. Adenine arabinoside effecton experimental idoxiuridine-resistant herpessimplex infection.InvestOphthalmolVisSci.1974;4:302---4.
22.XuH,AgrafiotisDK.Retrospectandprospectofvirtualscreening indrugdiscovery.CurrTopMedChem.2002;12:1305---20.
23.ChooQL,KuoG,WeinerA.AcDNAclonederivedfroma blood-bornenon-A,non-Bviral.Science.1989;4902:359---62.
24.HoofnagleJH,MullenKD,JonesB,etal.Treatmentofchronic non-A,non-BHepatitiswithrecombinanthumanalfainterferon. Apreliminaryreport.NEnglJMed.1986;25:1575---8.
25.ReichardO, AnderssonJ.Ribavirin:apossiblealternativefor thetreatmentofchronicnon-A,non-Bhepatitis.ScandJInfect Dis.1990;4:509.
26.ReichardO,AnderssonJ,SchvarczR,etal.Ribavirintreatment forchronichepatitisC.Lancet.1991;337:1058---61.
27.SherlockS.Antiviraltherapyforchronichepatitisinfection.J Hepatol.1995;23:3---7.
28.Reichard O, Schuarcz R, Weiland O. Therapy of hepatitis C:alpha interferon and ribavirin. Hepatology. 1997;3 Suppl. 1:108s---11s.
29.TraynorK.Twodrugsapprovedforchronichepatitisinfection. AmJHealthSystPharm.2011;13(1176):2011.
30.JesudianAB,Gambarin-GelwanM,JacobsonIM.Advancesinthe treatmentofhepatitisCvirusinfection.GatroenterolHepatol. 2012;8:91---101.
31.YauAH,YoshidaEM.HepatitisCdrugs:theendofthepegylated interferon era and theemergence ofall-oralinterferon-free antiviralregimens.Aconcisereview.CanJGastroenterol Hep-atol.2014;28:445---51.
32.KimDY,AhnSH,HanKH.EmergingtherapiesforhepatitisC.Gut Liver.2014;8:471---9.
33.Palmer M, RubinR, Rustgi V. Randomised clinicaltrial: pre-dosing with taribavirin before starting pegylated interferon vsstandardcombinationregimenintreatmentnaïvepatients withchronichepatitisCgenotype1.AlimentPharmacolTher. 2012;36:370---8.
34.DeClercqE,FieldHJ.Antiviralprodrugs-thedevelopmentof successfulprodrugstrategiesforantiviralchemotherapy.BrJ Pharmacol.2006;1:1---11.
35.HeimMH.Interferons andhepatitis Cvirus.Swiss MedWkly. 2012;142:1---13.
36.Pfeiffer JK, Kirkegaard K. Bottleneck-mediated quasispecies restrictionduringspreadofanRNAvirusfrominoculationsite tobrain.ProcNatlAcadSci.2007;14:5520---5.
37.RomanoKP,AliA,AydinC,etal.Themolecularbasisofdrug resistanceagainsthepatitisCVirusNS3/4Aproteaseinhibitors. PLoSPathol.2012;7:1---15.
39.MalcomB,LiuR, LahserF,etal. SCH503034,a mechanism-based inhibitor ofhepatitis C virus NS3 proteasesuppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrobial Agents Chemother.2006;3:1013---20.
40.RehmanS,AshfaqUA,JavedT.Antiviraldrugsagainsthepatitis Cvirus.GenetVaccinesTherapy.2011;11:1---10.
41.Recommendationsfortesting,managing,andtreatinghepatitis C;2014,December.http://www.hcvguidelines.org
42.EuropeanAssociationfortheStudyoftheLiver. Recommenda-tionsontreatmentofhepatitisC;2014.http://www.easl.eu/ newsroom/latest-news/easl-recommendations-on-treatment-of-hepatitis-c-2014
![Table 2 Summary of the recent treatment guidelines for HCV infection therapy [described by the American Association for the Study of Liver Diseases (AASLD), Infectious Disease Society of America (IDSA) and the International Antiviral Society (IASUSA)].](https://thumb-us.123doks.com/thumbv2/123dok_es/6159167.182110/6.1216.66.1159.119.842/treatment-guidelines-infection-american-association-infectious-international-antiviral.webp)